4.3. METHODS TO IDENTIFY ESCAPED SEABASS AND SEABREAM Fish escape from farm facilities as a consequence of technical and operational failures, leading to the escape of millions of seabream and seabass into the wild each year (Prevent Escape Compendium Chapter 2). Once in the wild, escaped seabream and seabass can survive for months, swimming away from their cages to nearby fish farms, fishing grounds, coastal habitats and local harbours, where they feed on natural prey and compete for natural resources with wild populations (Chapter 4.5, this compendium). Thus, escapees could have negative ecological and genetic consequences on wild fish populations and nearby farmed stocks through the spread of pathogens, interbreeding or resources competition (Naylor et al. 2005). Moreover, there is a large interaction between aquaculture and local fisheries where the latter benefit from farm- aggregated wild fish and farmed escapees. An increase of escapees in fisheries landings has been recorded during the last years in Mediterranean coastal areas, which is accompanied by a decrease both in price and mean size of individuals (Dimitriou et al. 2007). Cite this article as: Arechavala-Lopez P, Sanchez-Jerez P, Fernandez-Jover D, Bayle-Sempere JT, Black KD, Ladoukakis E, Somarakis S, Dempster T (2013) Methods to identify escaped seabass and seabream. In: PREVENT ESCAPE Project Compendium. Chapter 4.3. Commission of the European Communities, 7th Research Framework Program. www.preventescape.eu authors: Pablo Arechavala-Lopez 1 , Pablo Sanchez-Jerez 1 , Damián Fernandez-Jover 1 , Just T. Bayle- Sempere 1 , Kenny Black 2 , Emmanuel Ladoukakis 3 , Stelios Somarakis 4 & Tim Dempster 5 1 University of Alicante, Spain; 2 Scottish Marine Institute, UK; 3 University of Crete, Greece; 4 Hellenic Centre for Marine Research, Greece; 5 NOFIMA, Norway. INTRODUCTION www.preventescape.eu 123 ISBN: 978-82-14-05565-8

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

4.3. METHODS TO IDENTIFY ESCAPED SEABASS AND SEABREAM
Fish escape from farm facilities as a consequence of technical and operational failures, leading to the escape of millions of seabream and seabass into the wild each year (Prevent Escape Compendium Chapter 2). Once in the wild, escaped seabream and seabass can survive for months, swimming away from their cages to nearby fish farms, fishing grounds, coastal habitats and local harbours, where they feed on natural prey and compete for natural resources with wild populations (Chapter 4.5, this compendium). Thus, escapees could have negative ecological and genetic consequences on wild fish populations and nearby farmed stocks through the spread of pathogens, interbreeding or resources competition (Naylor et al. 2005). Moreover, there is a large interaction between aquaculture and local fisheries where the latter benefit from farm-aggregated wild fish and farmed escapees. An increase of escapees in fisheries landings has been recorded during the last years in Mediterranean coastal areas, which is accompanied by a decrease both in price and mean size of individuals (Dimitriou et al. 2007).
Cite this article as: Arechavala-Lopez P, Sanchez-Jerez P, Fernandez-Jover D, Bayle-Sempere JT, Black KD, Ladoukakis E, Somarakis S, Dempster T (2013) Methods to identify escaped seabass and seabream. In: PREVENT ESCAPE Project Compendium. Chapter 4.3. Commission of the European Communities, 7th Research Framework Program. www.preventescape.eu
authors:Pablo Arechavala-Lopez1, Pablo Sanchez-Jerez1, Damián Fernandez-Jover1, Just T. Bayle-Sempere1, Kenny Black2, Emmanuel Ladoukakis3, Stelios Somarakis4 & Tim Dempster5
1 University of Alicante, Spain; 2 Scottish Marine Institute, UK; 3 University of Crete, Greece; 4 Hellenic Centre for Marine Research, Greece; 5 NOFIMA, Norway.
INTRODUCTION
www.preventescape.eu 123
ISBN: 978-82-14-05565-8

124
To better understand these potential negative effects, it is imperative to distinguish and quantify the number of individuals that escape from sea cages. Effective tools which differentiate farmed individuals within wild stocks are required. These tools will improve scientific knowledge of escape events, help to assess potential genetic and ecological risks of escapees on wild populations, and help assess their contribution to fisheries landings. Furthermore, within the production chain, similar fish products can arise from different points of origin and there is potential for fraud due to product mislabelling. The temptation to label farmed fish as wild fish by fish merchants, retailers and restauranteurs is significant because of the price premium commanded by wild fish. Thus, verifiable and robust methods to distinguish farmed from wild fish are required for consumer confidence and for local authority enforcement purposes, to combat mislabelling and conform to legislation (Bell et al. 2007, Morrison et al. 2007).
Several techniques have been applied to classify salmon according to their farmed or wild origin, based on the assumption that wild and cultured salmon experience large differences in growth, feeding regimes and environments. This process started in the 1980s when, for a simple and quick identification, a combination of several techniques typically used for stock identification in fisheries were used routinely to survey the amount of farmed escapees in wild catches of salmon (Fiske et al. 2005). At present, a wide range of techniques (genetic, chemical characteristics, fatty acid composition, trace element levels, presence of pollutants, stable isotopes, morphology and organoleptic characteristics) are used to distinguish between wild and escaped farmed fish.
TOOLS TO IDENTIFY ESCAPEES
We tested a broad suite of tools to discriminate between the farmed or wild origin of seabream and seabass in the Mediterranean Sea. Our aim was to assess which technique could be effectively applied to accurately assess the level of escapee intrusion into wild populations and fisheries landings.
OBJECTIVE

125www.preventescape.eu
Different techniques were applied to differentiate between wild and farmed seabream and seabass from Spain and Greece, from the easiest and cheapest methods to the most labour-intensive or expensive.
IDENTIFICATION OF SEABREAM AND SEABASS ESCAPEES
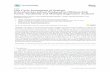
External appearance and morphological characteristics reflect to some degree the life history of the fish, since external characteristics in farmed fish are affected by culture conditions, such as stocking density and feeding strategy (Grigorakis 2007). Apart from the usual body malformations easily observable in some farmed fish (e.g. Sola et al. 1998, Loy et al. 1999), wild gilthead seabream exhibit lower body height than farmed seabream, which have a sharper snout and a more squat and compact shape (i.e. being shorter, wider and higher; Figure 4.3.1; Flos et al. 2002, Grigorakis et al. 2002). Farmed seabream had small, rounded and less developed teeth compared to the bigger, sharper teeth in their wild counterparts (Grigorakis et al. 2002). Skin characteristics also differ between wild and farmed fish; farmed seabream have thinner skin which is much darker in the dorsal and head areas. The characteristic iridescent colours of this species are much duller, which is suggested to be related to lack of access to natural, rather than commercial food (Grigorakis et al. 2002). Moreover, farmed seabream have a smaller belly and sharper dorsal fins and a higher degree of erosion in the caudal and pectoral fins (Grigorakis et al. 2002), which are strongly related to the stocking density and swimming behaviour of farmed seabream. In contrast, external differences in European seabass among wild and cultured fish are not as pronounced, and identification cannot rely on shape, colour, general appearance or fin erosion (Eaton 1996). However, we detected significant morphometric differences (through Truss Network System analysis; Figure 4.3.2) in the cranial and body regions of seabream and seabass relating to their farmed or wild origin. Furthermore, a higher condition index, which is a typical indicator of good dietary condition, was found for cultured fish compared to wild fish for both seabream and seabass. Other indices, such as relative profile for seabream and cephalic index for seabass, are good indicators of fish origin. Thus, wild and cultured seabream and seabass show external differences, which cannot only be used to indicate dietary condition and history
EXTERNAL APPEARANCE AND MORPHOLOGY

126
Figure 4.3.1. Wild (top) and farmed (bottom) gilthead seabream from the Spanish coast.
(Grigorakis 2007), but might also be easy, cheap and reliable tools to discriminate escapees from wild fish shortly after escape incidents have occurred. Therefore, we recommend morphological features as a first step to determining escapees. Such evaluations of fish origin could be made by farmers, fishermen, merchants, consumers, scientists or management agencies.

www.preventescape.eu 127
Figure 4.3.2. Example of landmarks and distances measured on seabream (top)
and seabass botoom) used to assess morphometric differences (Truss Network
System) in wild and farmed fish.

High fish densities within cages (5 - 10 kg m-3) lead to greater numbers of physical collisions and thus high friction among individuals. Pronounced levels of scale loss occur through abrasion (Eaton 1996), aggressiveness, lymphocystis or handling (Fiske et al. 2005). Logically, a high rate of scale detachment in farmed fish leads to a high rate of scale replacement. In comparison, scale loss and replacement in wild fish are relatively rare. We detected higher average levels of replacement scales in farmed seabream than wild fish; this can be used to classify seabream specimens to farmed or wild origin. Although fish can completely replace a scale within a year of its loss, wild fish scales are still distinguishable from those of farmed fish, which will never regenerate the nucleus (Figure 4.3.3). In addition, growth patterns are also reflected in scale patterns. Wild fish have a slower growth rate and show more annuli, and fewer esclerites, on their scales than farmed fish. This is a result of the more constant environmental conditions under which the fish are reared, combined with a regular food supply, which greatly reduces the effects of seasonality on the growth patterns of the fish (Figure 4.3.3). Therefore, scale characteristics are the easiest and quickest way to identify escapees. They can be used directly in the field by non-experts, without expensive equipment or labour intensive methods.
Environmental conditions, food supply and genetic dissimilarities can influence the shape of the sagittal otoliths (Pannella 1971), which could help distinguish the farmed or wild origin of seabream and seabass. In general, seabream sagittal otoliths had a pentagonal to elliptical shape with serrate margins, while seabass sagittal otoliths are fusiform to oblong in shape (Figure 4.3.4). In both species, the use of image analysis techniques, such as shape descriptors or elliptical Fourier analysis, and further discriminant analysis, detected differences based on wild or farmed origin with high accuracy, and also detected differences between geographical origins for fish of larger sizes. This methodology is a more objective, reliable method that the use of external morphometric traits, as they are not affected by short-term variations in fish physiological condition, standard tissue preservation techniques, or by geographical differences in morphology.
128
SCALES AND OTOLITHS SHAPE

www.preventescape.eu 129
Figure 4.3.4. Sagittal otoliths from wild and farmed seabream and seabass.
Figure 4.3.3. Scale characteristics of wild and farmed seabream and seabass.

In marine aquaculture, the high lipid content of the diet and the intensive feeding regime affect the chemical composition of the fish, resulting in a higher fat content (Lopparrelli et al. 2004). Fish lipids are well known to be rich in long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFA), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which together with arachidonic acid (ARA), are considered to be essential dietary fatty acids. The incorporation and storage of FAs in fish tissues strongly depend on the FA profile of the diet (Sargent et al. 2002). Hence, the current practice of substituting fish oils with other vegetable lipid sources in farmed marine fish diets leads to notable changes in lipid composition and FAs profiles, due to the presence of FAs of terrestrial origin such as oleic acid (OA), α-linolenic acid (LNA) or linoleic acid (LA), which are usually found in low levels in marine fish. Therefore, differences between wild and farmed dietary FA profiles have been widely used to discriminate farmed and wild fish origin.
Several authors have studied the differences in FA composition of different tissues, such as muscle, liver, skin or brain, in both seabream and seabass. Generally, these studies showed
Figure 4.3.5. Proportion of Linoleic acid and Arachidonic acid in farm and wild seabream muscle.
FATTY ACID PROFILES IN FLESH AND LIVER TISSUES
130
SEABREAM

pronounced differences between wild and farmed counterparts according to LA, which is found in higher proportion in farmed fish, and ARA, which is found in lower levels for adult cultured seabass and seabream (Figures 4.3.5 and 4.3.6). However, different patterns have been established for the rest of individual FAs between wild and farmed fish because of the high variability and high standard deviations within the reviewed results. Such high heterogeneity is strongly affected by the dietary history and exhibits strong seasonality (Grigorakis et al. 2007, Yildiz et al. 2008). To strengthen the reliability of the analysis of FA profiles, a multivariate approach (e.g. Principal Component Analysis), which takes into account the whole FA profile, is effective for differentiating wild and farmed seabream and seabass. We used a multivariate analysis to discriminate wild and cultured fish species according to their FA profile and this technique proved to be a strong tool.
The most important source of concern regarding this technique is the ‘wash-out’ of fatty acids due to the ability of escaped fish tofeed in the wild (Chapter 4.5, this compendium) and therefore substitute FAs from terrestrial origin with a FA from natural diets. The fatty acid signature may clearly reveal the origin of an individual if it has recently escaped from a fish farm after being feed with commercial pellets for a period of time. However, the FA profile of an escapee will change if a natural diet is consumed over time. In this case, other techniques need to be applied.
Figure 4.3.6. Proportion of Linoleic acid and Arachidonic acid in farm and
wild seabass muscle.
131www.preventescape.eu
SEABASS

132
Marine fish will incorporate different trace elements from the environment into their skeletal tissues and organs, either present in seawater or the diet, forming a chemical signature that will reflect the length of time that a fish has inhabited a particular water body (Lal 1989). Hence, trace element profiles are likely to be unique to a given population that inhabits one given location, and aquaculture creates a special situation in which the normally roaming species become fixed in one specific location with also unique environmental conditions.
Wild populations of seabass and seabream in the Mediterranean are known to roam between different zones, and for this reason it could be difficult to find differences in trace elemental signatures among otoliths from different populations of wild seabass and seabream. We studied trace elements in scales and otoliths and detected clear differences between wild and farmed seabream and seabass for specific elements. For instance, higher values of Mn and Ba, and lower levels of Sr, were found in farmed fish than in wild fish for both species (Figures 4.3.7 and 4.3.8). Despite the fact that other studies gave contrasting results, most were able to distinguish wild and farmed fish with great accuracy, but only through a multivariate approach which accounted for a wide range of elements. No simple diagnostic elemental concentration or ratio exists. This indicates that the trace elemental profile might be more appropriate than the presence or absence of a specific quantity of an element (Figures 4.3.7 and 4.3.8).
Figure 4.3.7. MDS plot of elemental analysis of scales from putatively wild (W) and farmed (F) seabream from 2 farms in Mediterranean Spain (Alicante). Stress factors are shown in corner boxes. Ellipses delimit 95% confidence intervals. Elements are ranked in terms of their contribution to the observed distribution.
TRACE ELEMENTS IN SCALES AND OTOLITHS

Figure 4.3.8. MDS plot of elemental analysis of scales from putatively wild (W) and farmed (F) seabass from 2 farms in Mediterranean Spain (Alicante). Stress factors
are shown in corner boxes. Ellipses delimit 95% confidence intervals. Elements are ranked in terms of their contribution to the observed distribution.
GENETIC DIFFERENCES
www.preventescape.eu 133
Identifying escapees with genetic methods may prove quick, reliable and applicable in different ontogenetic levels of fish. Molecular markers could be applied to characterize the genetic structure of wild and farmed populations. Provided that farmed and wild populations are genetically distinguishable and well-characterized, individual fish taken at random can be allocated in one or the other population with some probability based on their genetic profiles. We used a set of 16 polymorphic microsatellite loci for seabream and another 16 loci for seabass to study the genetic profile of farmed and wild populations from Spain and

134
Greece. All populations for both species showed a deficit in Hardy-Weinberg equilibrium. Gene diversity was slightly higher in wild than in farmed populations for both species. This is expected as farmed populations contain a small proportion of the total diversity of the wild populations. Average inbreeding coefficient (FIS) was smaller for seabream than for seabass. It was significantly higher than zero for all populations, except the Greek farmed population for both species. Pairwise FST coefficient was significantly higher than zero for all populations for both species. This suggests that all populations are genetically different from each other.
There are three different explanations for the genetic differentiation between farmed populations and the nearby wild populations. First, farmed populations have evolved under different conditions from the wild populations from which they were originated. This explanation is highly unlikely because there has not been enough time for such genetic differentiation and because there is continuous gene flow towards farmed populations as the breeders come from wild populations. Particularly for gilthead seabream, a sequential protandrous hermaphrodite species, there is a continuous need to introduce wild males into aquaculture since most individuals turn into females after their second year of reproduction. Second, breeders from the wild that are used in aquaculture are a small and non-random sample of the wild populations. Third, breeders or fry, which are used in a location, have been imported from another location. All three explanations might hold simultaneously, but with different significance for each of them. The genetic differentiation of farmed from wild population is also shown using Bayesian clustering. This method makes possible the identification of potential escapees to the environment (Glover et al. 2009). The probability of allocation of an individual to a certain population depends on the genetic differentiation of the populations. This probability was higher in the Greek populations for both species than in the Spanish populations (Figure 4.3.9 and 4.3.10).
Figure 4.3.9. Bayesian clustering of farmed and wild seabream for Greek and Spanish populations.

www.preventescape.eu 135
To assist in evaluating the potential risk on wild stocks due to farm escapees, we have identified several useful tools to identify the wild or farmed origin of fish. Techniques such as morphology, external appearance or fatty acid profiles are valuable methods to distinguish between the farmed and wild origins of seabream and seabass in a short-term period. However, how long these differences last once a farmed fish enters the wild, which will influence the accuracy of these methods over time, remains unknown. Scale characteristics are the quickest and cheapest way to identify escapees with a high accuracy in field studies, and are easy to use for farmers, fishermen, merchants, consumers, scientists or managers.
While effective tracing using these various methods will assist in identifying the levels of escapee intrusions into wild populations, an alternative is to mark all farmed fish in hatcheries before they are transferred to sea cages. Marking may enable, for the first time, direct tracing of escapees back to the farm and even cage of escape, which will assist in pinpointing how the escape incident first occurred and inform escape prevention methods (Chapter 2, this compendium). Marking of fish in ways that ensure mark retention throughout the life cycle of the fish, without adverse effects on growth and welfare, is an important area for future research.
Figure 4.3.10. Bayesian clustering of farmed and wild seabass for Greek and Spanish populations. populations.
DISCUSSION

The presence of a regenerated nucleus in seabream scales and the lack of annual rings on seabass scales are the easiest and quickest way to identify an escapee. We recommend these methods for applications where rapid assessments are required in the immediate days to weeks after an escape event has occurred.
A multivariate approach using chemical and/or molecular characteristics represents a highly accurate method for distinguishing farmed seabream and seabass from their wild counterparts. We recommend these methods are used by management agencies and scientists for applications where a high degree of accuracy is required and escapes need to be detected many months after an escape incident has occurred.
RECOMMENDATIONS
136

www.preventescape.eu 137
Arechavala-Lopez P, Sanchez-Jerez P, Bayle-Sempere JT, Sfakianakis DG, Somarakis S (2012a) Morphological differences between wild and farmed Mediterranean fish. Hydrobiologia 679:217–231
Arechavala-Lopez P, Sanchez-Jerez P, Bayle-Sempere JT, Sfakianakis DG, Somarakis S (2012b) Discriminating farmed fish from wild Mediterranean stocks through scales and otoliths. J Fish Biol (in press)
Arechavala-Lopez P, Sanchez-Jerez P, Izquierdo-Gomez D, Toledo-Guedes K, Bayle-Sempere JT (2012c) Fin erosion on wild and farmed Sparus aurata (L.) and Dicentrarchus labrax (L.). J Appl Ichthyol (in press)
Bell JG, Preston T, Henderson RJ, Strachan F, Bron JE, Cooper K, Morrison DJ (2007) Discrimination of wild and cultured European seabass (Dicentrarchus labrax) using chemical and isotopic analyses. J Agri Food Chem 55:5934-5941
Dempster T, Moe H, Fredheim A, Jensen Ø, Sanchez-Jerez P (2007) Escapes of marine fish from sea-cage aquaculture in the Mediterranean Sea: status and prevention. CIESM Workshop Monogr 32:55–60
Dimitriou E, Katselis G, Moutopoulos DK, Akovitiotis C, Koutsikopoulos C (2007) Possible influence of reared gilthead seabream (Sparus aurata L.) on wild stocks in the area of the Messolonghi lagoon (Ionian Sea, Greece). Aquacult Res 38:398–408
Eaton DR (1996) The Identification and Separation of Wild-caught and Cultivated Seabass (Dicentrarchus labrax). MAFF Fisheries Research Technical Report, vol. 103. MAFF, Lowestoft, 15 pp
Fiske P, Lund RA, Hansen LP (2005) Identifying fish farm escapees. In Stock Identification Methods: Applications in Fishery Science Ed: Cadrin, S. X., K. D. Friedland & J. R. Waldman / Elsevier, Amsterdam. pp 659–680
Flos R, Reig L, Oca J, Ginovart M (2002) Influence of marketing and different land-based system on gilthead seabream (Sparus aurata) quality. Aquacult Int 10:189–206
Gillandres BM, Sanchez-Jerez P, Bayle-Sempere JT, Ramos-Esplá A (2001) Trace elements in otoliths of the two-banded bream from a coastal region in the south-west Mediterranean: are there differences among locations? J Fish Biol 59:350–363
REFERENCES CITED
LINKS TO PUBLISHED DOCUMENTS

138
Glover KA, Hansen MM, Skaala O (2009) Identifying the source of farmed escaped Atlantic salmon (Salmo salar): Bayesian clustering analysis increases accuracy of assignment. Aquaculture 290:37–46
Grigorakis K (2007) Compositional and organoleptic quality of farmed and wild gilthead seabream (Sparus aurata) and seabass (Dicentrarchus labrax) and factors affecting it: a review. Aquaculture 272:55–75
Grigorakis K, Alexis MN, Taylor KDA, Hole M (2002) Comparison of wild and cultured gilthead seabream; composition, appearance and seasonal alterations. Int J Food Sci Tech 37:477–484
Lal SP (1989) Minerals. In Fish nutrition. Ed: J. E. Halver /Academic Press San Diego. pp 220–257
Loy A, Boglione C, Cataudella S (1999) Geometric morphometrics and morpho-anatomy: a combined tool in the study of seabream (Sparus aurata, Sparidae) shape. J Appl Ichthyol 15:104–110
Morrison J, Preston T, Bron JE, Henderson RJ, Cooper K, Strachan F, Bell JG (2007) Authenticating production origin of gilthead seabream (Sparus aurata) by chemical and isotopic fingerprinting. Lipids 42:537–545
Naylor R, Hindar K, Fleming IA, Goldburg R, Williams S, Volpe J, Whoriskey F, Eagle J, Kelso D, Mangel M (2005) Fugitive salmon: assessing the risks of escaped fish from net-pen aquaculture. BioScience 55(5):427–437
Pannella G (1971) Fish otoliths: daily growth layers and periodical patterns. Science 173:1124–1127
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Fish nutrition. Ed: Halver JE, Hardy RW / Academic Press, San Diego, CA
Sola L, De Innocentiis S, Rossi AR, Crosetti D, Scardi M, Boglione C, Cataudella S (1998) Genetic variability and fingerling quality in wild and reared stocks of European seabass. In Bartley, D. & B. Basurco (eds). Cah Opt Méd 34:273–280
Yildiz M, Sener E, Timur M (2008) Effects of differences in diet and seasonal changes on the fatty acid composition in fillets from farmed and wild seabream (Sparus aurata L.) and seabass (Dicentrarchus labrax L.). Int J Food Sci Tech 43:853–858
Related Documents