1. Distinguish between a cation and an anion. 2. Which elements tend to form cations? anions? 3. Using a periodic table, predict the charge ion each of the following elements form: a. calcium c. sulfur b. fluorine d. aluminum Warm Up : December 4 th

1.Distinguish between a cation and an anion. 2.Which elements tend to form cations? anions? 3.Using a periodic table, predict the charge ion each of the.
Dec 16, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

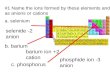
1. Distinguish between a cation and an anion.
2. Which elements tend to form cations? anions?
3. Using a periodic table, predict the charge ion each of the following elements form:
a. calcium c. sulfurb. fluorine d. aluminum
Warm Up: December 4th

Writing Formulas and Naming Chemical Compounds
• There are two types of chemical compounds:– ionic compounds– covalent (molecular) compounds
• Ionic Compounds are composed of a cation and an anion.– cations – positively charged ion (metals)– anions – negatively charged ion (nonmetals)

Monoatomic Ions• Cations
– For monatomic cations, the name of the ion is the name of the element.
– examples:
Na+ = sodium ion
Mg2+ = magnesium ion
Al3+ = aluminum ion

– Some metals are able to form more than one stable ion (polyvalent)
• usually transition elements• example: copper • forms Cu2+ and Cu+
• use Roman numerals to distinguish charge• copper (II) and copper (I)

• Anions– Monoatomic anions are named by dropping the
ending of the element and replacing with the suffix “-ide”.
– examples:
Cl- = chloride ion
O2- = oxide ion
N3- = nitride ion

Naming Binary Ionic Compounds
• The nomenclature, or naming system, or binary ionic compounds involves combining the names of the compound’s positive and negative ions.
• The name of the cation is given first, followed by the name of the anion:– example: Al2O3 — aluminum oxide

Independent practice
1. What is the formula and name for Sodium and Oxygen?
2. What would the formula and name be for potassium and sulfur?

The Stock System of Nomenclature
• Some elements such as iron, form two or more cations with different charges (polyvalent).
• To distinguish the ions formed by such elements, scientists use the Stock system of nomenclature.
• The system consists of a Roman numeral placed in parentheses after the metal name to indicate an ion’s charge.– examples: Fe2+ iron(II)– Fe3+ iron(III)

The Stock System of Nomenclature
Write the formula and give the name for the compound formed by the ions Cr3+ and F–.

Independent Practice
1. Give the formula for Chromium (II) Fluoride.
2. What is the name of Fe2O3?

Compounds Containing Polyatomic Ions
• Many common polyatomic ions are negatively charged and oxyanions— polyatomic ions that contain oxygen.
• Some elements can combine with oxygen to form more than one type of oxyanion.– example: nitrogen can form or .
3NO
nitrate nitrite
• The name of the ion with the greater number of oxygen atoms ends in -ate. The name of the ion with the smaller number of oxygen atoms ends in -ite.
2NO
3NO2NO

• Some elements can form more than two types of oxyanions.– example: chlorine can form , ,
or .
• In this case, an anion that has one fewer oxygen atom than the -ite anion has is given the prefix hypo-.
• An anion that has one more oxygen atom than the -ate anion has is given the prefix per-.
hypochlorite chlorite chlorate perchlorate
ClO2ClO
3ClO4ClO
ClO2ClO
3ClO
4ClO

Understanding Formulas for Polyatomic Ionic Compounds

Give the formula for…
1. Potassium Chlorate
2. Hydrogen Sulfite
3. Tin(IV) sulfate.

One Minute Paper• You have one minute to answer these two questions
concerning today’s lesson.
– What was the most important thing you learned?
– What is one question you would still like answered?
Related Documents