BIOCHEMISTRY // CELL AND CELL MEMBRANE Page 1 of 16 BIOCHEMISTRY - It is the frontier of science. It is not a dead science. There are still many things unexplored in biochemistry. - Example: Human genome - Published in 1986 and only 1-2% of 33,000 enzymes working with billions of base pairs of genomes are used and studied. - Biochemistry goes hand in hand with molecular biology. Others call it physiological chemistry or the chemistry of life. - Understanding the biochemical aspect of cell and cell membrane gives us the basis for what comes out as disease or those that do not. Phylogeny of the Three Distinct Domains of Life A. Archaebacteria Extreme halophiles (salt-tolerant) Methanogens Extreme thermophiles B. Eubacteria Thermatoga Flavobacteria Cyanobacteria Purple Bacteria Gram-positive bacteria Green nonsulfur bacteria C. Eukaryotes Animals Plants Ciliates Fungi Flagellates Microsporidia The macromolecule which is the first biochemical basis of life: RNA (ribonucleic acid) – Biohemical bases: 1. Informational capability to produce framework of protein 2. Protein needs to be acted upon by enzyme Cell Introduction Classification of All Organisms according to Energy Source and Source of Carbon A Comparison of Prokaryotes and Eukaryotes Prokaryotes Eukaryotes Organism 1-10 μm in size i.e. Eubacteria, Archaebacteria 10-100 μm i.e. Fungi, Plants, Animals Form Single-celled Single or multicellular Organelles, cytoskeleton, cell division apparatus Missing Present, complicated, specialized DNA Small, circular (ALL, with the exception of viruses which can have linear DNA), no introns, plasmids Large, in nucleus, many introns (evident difference from prokaryotes) *introns – non-coding sequence, not translated RNA: Synthesis and maturation Simple, in cytoplasm Complicated, in nucleus Protein: Synthesis and maturation Simple, coupled with RNA synthesis Complicated, in the cytoplasm, and the rough ER Metabolism Anaerobic (i.e. fermentation) or aerobic, very flexible Mostly aerobic, compartmented Endocytosis and exocytosis No *enveloped by a thick cell wall Yes Differentiation between an Animal and Plant Cell Animal Cell Plant Cell Nucleolus + + Nucleus + + Nuclear membrane + + ER (rough, smooth) + + Golgi apparatus + + Ribosomes + + Peroxisomes + - Lysosomes + - Cytoskeleton + + Plasma membrane + + Vacuole - + Cell wall - + Chloroplasts -, counterpart is the mitochondria + Starch granules - + Thylakoids - + Plasmodesma -, counterparts are gap junctions, etc. + Glyoxysome -, lysosomes as counterpart + BIOCHEMISTRY Trans No.: 1 CELL AND CELL MEMBRANE: A BIOCHEMICAL APPROACH Lecturer: Dr. Allan Hilario Date: June 10, 2013 Transcribed by: Balondo, Cahanding, dela Cruz, Dominguez, Farillas, Frias, Montes, Uichanco

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 1 of 16
BIOCHEMISTRY
- It is the frontier of science. It is not a dead science. There are still many things unexplored in biochemistry.
- Example: Human genome
- Published in 1986 and only 1-2% of 33,000 enzymes working with billions of base pairs of genomes are used and studied.
- Biochemistry goes hand in hand with molecular biology. Others call it physiological chemistry or the chemistry of life.
- Understanding the biochemical aspect of cell and cell membrane gives us the basis for what comes out as disease or those that do not.
Phylogeny of the Three Distinct Domains of Life
A. Archaebacteria
Extreme halophiles (salt-tolerant)
Methanogens
Extreme thermophiles B. Eubacteria
Thermatoga
Flavobacteria
Cyanobacteria
Purple Bacteria
Gram-positive bacteria
Green nonsulfur bacteria C. Eukaryotes
Animals
Plants
Ciliates
Fungi
Flagellates
Microsporidia The macromolecule which is the first biochemical basis of life: RNA (ribonucleic acid) – Biohemical bases:
1. Informational capability to produce framework of protein 2. Protein needs to be acted upon by enzyme
Cell Introduction
Classification of All Organisms according to Energy Source and Source of Carbon
A Comparison of Prokaryotes and Eukaryotes
Prokaryotes Eukaryotes
Organism 1-10 µm in size i.e. Eubacteria, Archaebacteria
10-100 µm i.e. Fungi, Plants, Animals
Form Single-celled Single or multicellular
Organelles, cytoskeleton, cell division apparatus
Missing Present, complicated, specialized
DNA
Small, circular (ALL, with the exception of viruses which can have linear DNA), no introns, plasmids
Large, in nucleus, many introns (evident difference from prokaryotes)
*introns – non-coding sequence, not translated
RNA: Synthesis
and maturation
Simple, in cytoplasm
Complicated, in nucleus
Protein: Synthesis
and maturation
Simple, coupled with RNA synthesis
Complicated, in the cytoplasm, and the rough ER
Metabolism
Anaerobic (i.e. fermentation) or aerobic, very flexible
Mostly aerobic, compartmented
Endocytosis and
exocytosis
No *enveloped by a thick cell wall
Yes
Differentiation between an Animal and Plant Cell
Animal Cell Plant Cell
Nucleolus + +
Nucleus + +
Nuclear membrane
+ +
ER (rough, smooth)
+ +
Golgi apparatus
+ +
Ribosomes + +
Peroxisomes + -
Lysosomes + -
Cytoskeleton + +
Plasma membrane
+ +
Vacuole - +
Cell wall - +
Chloroplasts -, counterpart is the
mitochondria +
Starch granules
- +
Thylakoids - +
Plasmodesma -, counterparts are gap junctions, etc.
+
Glyoxysome -, lysosomes as
counterpart +
BIOCHEMISTRY Trans No.: 1
CELL AND CELL MEMBRANE: A BIOCHEMICAL APPROACH
Lecturer: Dr. Allan Hilario Date: June 10, 2013
Transcribed by: Balondo, Cahanding, dela Cruz, Dominguez, Farillas, Frias, Montes, Uichanco
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 2 of 16
Structure of an Animal Cell
*Left box (yellow) – percentage of the cell *Right box (blue) – quantity in the cell *Number of mitochondrion depends on the differentiation function of the cell
A. Isolation of Cell Organelles
Fractional Centrifugation – way to differentiate the compartments of the cell 1. Lyse the tissue 2. Sieve it through a filter membrane to remove the
debris like the whole tissue part 3. First to sediment are the nucleus and the
cytoskeleton 4. Second are the organelles – mitochondria,
lysosomes, peroxisomes; plants – chloroplasts 5. Third, plasma membrane and ER fragments, small
vesicles, microsomal fraction 6. Last, ribosomes, viruses, macromolecules
(polymers of carbohydrates, proteins, etc.)
B. Density Gradient Centrifugation
A way to differentiate by gradient. When gradient is known of a particular solution, the particular cellular component will hover within that gradient, then you can know its identity by using its marker molecules
Ex. Peroxisome contains catalase (can attach a probe with an antibody against catalase, and find it using ELISA)
Prokaryotic cell
Ex. E. coli
Nucleodis not encapsulated
The bacteria maintains its shape through the cell wall which contains lipopolysaccharide (LPS) which is a membrane bound lipid (Lipid A) attached to polysaccharides which act as endotoxin, responsible for the symptoms of fever and shock in infected animals and humans by gram (-) bacteria. (septicemia)
Gram (+) Gram (-)
Color Blue-violet Pink
Stain Crystal Violet (Primary)
Safranin
Crowded inside with ribosomes and different solutes it makes it easier for solute to communicate, anything that comes in the cell is readily attached to any part of the cell targeted
No membranes separating the organelles
The plasmid is a different set of gene, usually circular which conveys resistance (the material that jumps from cell to cell to prevent destruction by antibiotics)
A. Components of a Bacterial Cell
Water – 60-70%
Water interacts with most substances in the cell making its interaction with water, a classifying distinction for these substances (Hydrophobic vs. hydrophilic)
The cell is enveloped by cell membrane serving as a selective physical barrier
The cell is composed of supramolecular structures capable of self-assembly
B.Inner look
Inner space of cell is crowded with macromolecules provide non-specific stearic repulsion prevents easy introduction of molecules inside
Macromolecules occupy 20-40% (protein is dominant)
Smaller molecules 70% of the space
Crowding affects:
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 3 of 16
o Biochemical reaction rates o Protein folding o Protein-protein binding o Chromosome structure o Gene expression o Signal transduction
*Why crowding – takes less time to interact due to less distance between cells
Proteins becomes super complexes o act as biological machines that perform work
when motor protein subunits bind and hydrolyze nucleotides such as ATP
o ATP – universal currency of cells
Energy-induced changeproduce conformational and orderly change in shape of the adjacent subunits causing movement (work)
All cells have the inherent ability to interpret and response to signal from its environment and adjacent cells through signal transduction (ability of the cell to respond to stimuli from the environment)
C. Biochemical Reactions of the Cytoplasm
Pentose phosphate pathway
Gluconeogenesis
Protein biosynthesis
Fatty acid biosynthesis
Glycolysis, etc. *In prokaryotes, everything happens in the cytoplasm “These characteristics are recurring theme in both prokaryotes as exemplified by a bacterial cell and eukaryotes as exemplified by an animal cell.”
Metabolism is the totality of cellular activities that
generates energy for cellular function, (1) conversion of micronutrients, (2) polymerization of various monomeric components of macromolecules and (3) synthesis and degradation of various macromolecules various and specialized cellular functions.
Anabolism is the synthesis of macromolecules from its
monomeric units usually for storage. - needs reducing equivalents
Catabolism is the degradation of macromolecules to its
monomeric units usually for energy production, entrance to other metabolic pathways or disposal.
- an oxidative process
CELLULAR ORGANIZATION Eukaryotic cell
- Coming from the three germ layers: 1. Ectoderm – producing the external 2. Mesoderm – mesenchymal 3. Endoderm – mostly neural tissue
- There are over 200 cell types in the human body.
TWO MAJOR PARTS OF THE CELL
1. Cytoplasm
- Portion of the cell enclosed by the cell membrane and outside the nucleus
- Where the cellular organelles are found which are membrane-bound or the macromolecules and other ribosomes which are not membrane-bound
- These organelles are not just simply floating but are linked together by the cytoskeleton of the cell
- Cytosol: fluid portion of the cytoplasm; where most of the
biochemical processes occur - These include glycolysis, gluconeogenesis, fatty acid
synthesis, protein synthesis, part of urea cycle and purine metabolism an amino acid synthesis, NADPH production (pentose phosphate pathway; malic enzyme), isoprenoid and sterol synthesis (early stages), fatty acid synthesis
- But not all cells will do that. There are differentiation of cells where only gluconeogenesis is present and ketogenesis is absent.
- Nucleoplasm: the cytosol in the nucleus
Cytoskeleton
- a network of protein scaffolding - maintains the shape of the cell
- serves as tracts for movement of organelles - These filamentous protein polymers are categorized into
3 types depending on its size with differing functions: 1. Microfilaments (6-8 nm) 2. Intermediate filaments (10 nm) 3. Microtubules (25 nm)
- The protein scaffolding assembles as polymers from protein subunits.
- What is that recurring theme from the prokaryotes to the eukaryotes? SELF- ASSEMBLY (They have the same characteristics that make them assemble. The non-covalent factors that make them assemble: London forces, stearric, Van der Waals, hydrogen bond, dipole-dipole, ionic bond.)
1. Microfilaments - Actin
most abundant protein in eukaryotic cells the protein component of microfilaments occurs in two forms: a monomolecular form (G-
actin, globular actin) and polymer (F-actin, filamentous actin)
G-actin is an asymmetrical molecule with a mass of 42 kDa, consisting of two domains.
As the ionic strength increases, G actin aggregates reversibly to form F actin, a helical homopolymer. G actin carries a firmly bound ATP molecule that is slowly hydrolyzed in F actin to form ADP. Actin therefore also has enzyme properties (ATPase activity: It hydrolyzes ATP to ADP and organic phosphate).
Myosin is the globular protein that interacts with actin in myocytes for muscle contraction.
It is present in all cells not just in the muscles.
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 4 of 16
(Refer to the figure above) This is the single monomer of actin and when they polymerize, they become F actin. The negative end which is actually negative in its electric capacity is inhibited by phalloidin, a toxin. The positive end is inhibited by cytochalasin. These two can be used to prevent the formation of actin so the cell will die, especially in cancer cells.
2. Intermediate filaments
- The intermediate filament belongs to five related protein family with cell type-specificity. These include:
1. Cytokeratins (ectoderm) 2. Desmin (ectoderm) 3. Vimentin (mesoderm) 4. Glial fibrillary acidic protein (GFAP) (endoderm;
in the glial cells of the nervous tissue)
5. Neurofilament (endoderm) - These proteins have a rod-shape structure at the
center called super helix configuration. - The intermediate filament is produced from 8
protofilaments.
3. Tubulins
- The basic components of the tube-shaped microtubules are α– and β–tubulin (53 and 55 kDa).
- Thirteen protofilaments form a ring-shape and polymerize to form a long tube.
- Important during mitosis.
2. Nucleus
Largest organelle
Repository and cellular localization of storage replication and expression of genetic information
Contains 99%of cell DNA (1% in the mitochondria)
Structure: 1. Nuclear Envelope- separates the nucleus from the
cytoplasm; hastwo separate bilayer membranes (one inside the other) - The outer membrane is continuous w/ the ER of the
cell cytoplasm
- The space between the two membranes is continuous with the space inside the ER
2. Nuclear Pores- controls the movement of substances
between the nucleus and cytoplasm 3. Nucleolus- contains RNA and proteins of the types found
in ribosomes - Has no limiting membrane
4. Nucleoplasm- highly viscous liquid surrounding the
chromosomes and nucleoli 5. Chromatin- composed of DNA and other proteins that
make up the nucleus 6. Chromosomes- contains most of the cell’s hereditary
units (genes) that controls cell structure and its activities
Functions:
- Control center of the cell
- Contains large amounts of DNA - Transmits genetic information - Directs protein synthesis in cytoplasm via various RNA
forms - Contains enzymes for DNA repair, replication,
transcription, and mRNA production
Pathways that occurs in the nucleus:
1. Replication a. Nucleus -99% b. Mitochondria -1%
2. Transcription 3. Translation 4. Biosynthesis of NAD
+
Nucleolus- ONLY part where rRNA is synthesized Euchromatin- transcribing part; white part in the electron-
micrograph Heterochromatin -dense part; dark part
ORGANELLES
1.Mitochondria
- Powerhouse of the cell where, most not all, ATP is produced
- Self-replicating
Toxin found in Amanita phalloides mushroom inhibits decay by binding to the negative polarity.
Mold toxin inhibits polymerization by binding to the positive polarity
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 5 of 16
- Primitive prokaryotic cell engulfed the bacterium (animal-aerobic; plant-cyanobacterium) which resulted to the double-membrane structure called mitochondria (for animal and chloroplast for plants).
- Explains why mitochondria contains circular DNA which is also present in the bacterium
Stuctures:
1. Outer membrane –porous 2. Inner membrane – selectively porous 3. Cristae-where electron transport chain and oxidative
phosphorylation occur; contains enzymes required for ATP synthesis
4. Intermembrane space 5. Matrix
Proteins-major macromolecule content of mitochondria Pathways in the mitochondria:
1. Tricarboxylic Acid Cycle (Kreb’s Cycle) 2. Beta-Oxidation 3. Part of the Urea Cycle 4. Respiratory Chain 5. ATP Synthesis
Transport Systems in the Mitochondria:
1. Citrate-Malate Shuttle 2. Carnitine Shuttle 3. Glyerophosphate huttle 4. Malate shuttle
2. Rough and Smooth Endoplasmic Reticulum
A. Rough ER: Granular Endoplasmic Reticulum
- Flattened sacs of ER studded with ribosomes (where protein synthesis occurs) giving its rough appearance
Functions:
- Protein synthesis via ribosomes
- mRNA translational and post-translational modification of proteins
B. Smooth ER: Granular Endoplasmic Reticulum
- Tubular sacs of ER with no ribosomes attached
Functions:
- Site of lipid and cholesterol synthesis
- Drug detoxification - Calcium reservoir for cell signaling - Conversion of hydrophobic into water soluble molecules
*phospholipids produced in SER are immediately incorporated in the bilayer of the SER itself for the formation of transport /ER vesicles and replenish the cell membrane bilayer
3. Golgi Apparatus
Responsible for the packing of proteins in vesicles to be exported out of the cell
1. cis face - fusion of areas of Golgi with ER 2. trans face - site for packaging and sorting
Functions:
- Receives protein from rER via coated vesicles to be further processed, packed and released into the cytoplasm as vesicles
- Synthesizes certain carbohydrates not formed in the ER such as hyaluronic acid and chondroitin sulfate
4. Lyososomes
Contains nucleases, proteases, glcosidases, lipases, phosphatases, sulfatases, phospholipases
All macromolecules that gets into the lysosomes (very acidic in nature) are either catabolised for salvage usage or for waste disposal
Structures:
- Originates from the Golgi apparatus - Acidic at pH 4.5 - Membrane bound vesicles dispersed in the cytoplasm - Contains digestive enzymes (hydrolase) and bactericidal
agents (ex. lysozyme and lysoferrin)
Functions:
Degradative via endocytosis towards: a. Damaged cell structures b. Food particles ingested c. Unwanted materials (ex. bacteria)
- Responsible for tissue regression after long periods of
inactivity - Responsible for autolysis after cell damage
5. Peroxisomes
Degrades peroxides because of the enzyme oxidases present in it
Structures:
- Membrane bound - Forms by self replication
- Budding off from the sER - Contains oxidases instead of hydrolases
Functions:
Membrane-bound vesicles containing oxidative enzymes for: - Detoxifying harmful substances - Oxidation of fatty acids
Pathways in Peroxisomes:
1. Fatty Acid Oxidation (alpha, beta, omega) 2. Synthesis of certain membrane lipids 3. Purine catabolism 4. Generation and breakdown of NaOH
CELL MEMBRANE
Emerged with most polar charged substances but allows the hydrophobic substances or the non polar substance
A 5 to 10 nanometers in thickness
Appears trilaminar structure when viewed in electron microscope.
1. Trilaminar
- Actual picture of the lipid bilayer under the electron microscope
- Dense part of phosphate group in phospholipid - Less dense is the tail of the phospholipid
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 6 of 16
- The other dense part is the other polar group in the inner layer
2. Lipid Bilayer
Characteristic of the membrane
FLUID MOSAIC MODEL
1972 by Singher and Nicolson
Lipid bilayer wherein the lipid layer is DYNAMIC. There is a lot of movement in the inner and outer leaves.
Proteins and other structures in the bilayer moves. They “float in a sea of lipids.”
Integral membrane proteins are inserted into the lipid bilayer, whereas peripheral proteins arebound to the membrane indirectly by protein-protein interactions. Most integral membraneproteins are transmembrane proteins, with portions exposed on both sides of the lipid bilayer.The extracellular portions of these proteins are usually glycosylated, as are the peripheralmembrane proteins bound to the external face of the membrane.
LIPID CONTENT OF PLASMA MEMBRANE
Major content of the lipid bilayer is PHOSPHOLIPIDS
POLAR: Phosphate group; “head”
NON-POLAR: Fatty acyl chain; “tail”
Arranged in such a way that the outer polar group is in contact with the outer matrix and the inner polar group is in contact with the cytoplasm.
Middle contains the non-polar acyl chains of both tails.
They are attached by non-covalent bonds due to their stearic attraction.
In general, most commonly found are: Phospholipids, sphingolipids and cholesterol.
*Is the lipid content of plasma membrane constant in all cell types? No, they vary.
CELL MEMBRANE MOTION STUDIES I. LIPID MOVEMENT
1. The cell was reacted with fluorescent probe to label the
lipids of the cell membrane (first step). 2. Second step shows the fluorescent labeled cell
membrane and its appearance in the fluorescence microscope.
3. In the third step, an intense laser beam bleaches a small area of the cell membrane and its appearance microscopically.
4. In time, the unbleached phospholipids diffuse into the bleached area (therefore, movement).
II. PROTEIN MOVEMENT
1. Cells from two different species (human and mouse, blue
and pink respectively) are tagged with fluorescent antibodies that react with membrane proteins.
2. These cells are induced to fuse by exposure to Sendai virus. The proteins from the two parent cells are localized to two separate halves of the hybrid cell initially.
3. Within a few minutes, they begin to intermix. 4. After around 40 minutes, they are now randomly
distributed in the plasma membrane.
PROTEINS IN THE CELL MEMBRANE
GLYCOPROTEINS: outer leaflet Oligosaccharides: outer leaflet
Important for signal transduction and recognition
Antigen-antibody binding
FUNCTIONS OF THE CELL MEMBRANE
The bounding function of the membrane gives the cell its shape and separates itself from other cells and external environment.
When there is actual anchorage of any protein in the cell, there is less movement.
CELL MEMBRANE LIPID COMPOSITION
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 7 of 16
It can be generalized that the inner leaflet contains phosphatidylserine.
Tricylglycerol or neutral fat is not present in cell membranes with only a few exceptions.
Cholesterol is exclusively found in animal cell membranes. When present in plants, they are called steroids.
CELL MEMBRANE COMPOSITION IN DIFFERENT CELLS
TYPES
NERVE CELL PLASMA MEMBRANE o Sphingomyelin is high because it serves as an
insulator (i.e. myelin sheath)
MITOCHONDRIA o high in proteins because these proteins are enzymes
responsible for oxidative phosphorylation and the electron transport chain CARDIOLIPIN
only specialized lipid that is antigenic; present in the mitochondria
proof of endosymbiosis
SELF-ASSEMBLY OF PHOSPHOLIPIDS
Phospholipids when placed in an aqueous solution can assemble itself in different ways.
1. Micelle
- Hydration of polar heads and the tails aggregate in the center
- No cavity inside - Individual units are wedge-shaped (cross section of
head greater than that of side chain)
2. Lipid bilayer
Basic structural element of the cell membrane.
3. Vesicle/Liposome
Two layers of lipid bilayer engulfing an aqueous environment.
Presence of an aqueous cavity inside the inner membrane.
CELL MEMBRANE FLUIDITY
The most important characteristic of the fluid mosaic model is its fluidity.
Fluidity refers to the viscosity of the lipid bilayer or the degree by which the bilayer resists movement.
Factors Affecting Membrane Fluidity
1. Temperature
Higher temp, higher fluidity
2. Unsaturated Fatty Acid Content
More unsaturated fat, more fluid
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 8 of 16
Presence of double bonds, therefore kinks in hydrophobic tail.
The kinks can’t provide an ordered structure by stacking.
3. Cholesterol (paradoxical)
Aliphatic: polar and nonpolar
Paradoxical way by which it provides fluidity.
Cell Membrane Fluidity
Viscosity of the lipid bilayer or the degree of resistance of membrane components to move. Factors:
1. Temperature: higher T, more fluid 2. Content of unsaturated FA: higher content, more fluid 3. Content of cholesterol: when cholesterol interacts with
unsaturated/short-chain fattyacyl PL results to less fluidity; when cholesterol interacts with spingolipids and long-chain fattyacyl PL tends to make it more fluid.
Cell Membrane Lipid Movement
1. Rotation
2. Lateral diffusion - very fast
3. Flip flop - very slow that enzymes are needed
Components of phospholipid that needs enzyme:
1. Flippase - P-type ATPase - Allows one polar head group to go the other side - Requires energy
2. Floppase - ABC transporters - Inner leaflet to the other side - Requires energy
3. Scramblase - Moves either way
Cell Membrane Self-Sealing
This is for the maintenance of cell membrane integrity. Two types: a. Lateral diffusion of lipid contents for small disruptions b. Energy requiring process through Ca2+ for larger tear
due to mechanical stress
Cell Membrane Selective Permeability
The hydrophobic hydrocarbon chains in lipid bilayer provide an impervious barrier for ionic and polar substances.
Specific proteins - regulate the passage of ionic and polar substances in and out of the cell so that polar groups cannot pass through easily the cell membrane but hydrophobic groups can easily diffuse:
Water - aquaporins
Polar substances - shed hydration shell
Large molecules and ions - transport system such as protein membrane
Cell Membrane Asymmetry
The inner and outer leaflets of the lipid bilayer differ in composition
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 9 of 16
Cell Membrane Proteins
These are for transfer of solute. The latter cannot enter the lipid bilayer because it is polar.
Classification:
1. Integral proteins
- Embedded in and/or pass through the lipid bilayer - Extracted only through disruption of the cell membrane
with organic solvents or detergents - Amino group in cytoplasm or extracellular side
- Traverse the membrane in a certain manner wherein the hydrophobic part of the segment of the polypeptide traverse in certain type of manner the cell membrane making a ionic channel wherein solutes can pass through
- Allow hydrophobic interaction with lipid bilayer - Most have alpha-helical structures with 15 to 20 aa
- Have bulky side chains: alanine, leucine, isoleucine, phenylalanine, and valine
- May have several helices that pass through the membrane in serpentine manner
2. Transmembrane proteins (but not integral proteins)
- Beta-pleated sheet is the secondary structure: a. FepA-ion transport for bacteria b. OmpLA-phospholipase in E. coli c. Maltoporin- transport in E.coli d. In human: porins in outer membrane of
mitochodria
3. Peripheral proteins - Bound to the membrane through non-covalent
interaction with integral proteins - Covalently bound to several mechanism: myristic,
palmitic, prenyl group, glycosylphosphatidylinositol anchors (GPI-anchors) in lipid rafts
- Can also be found in extracellular
Hydropathy plot - a graph determining the sequence of amino
acid in polypeptide will have a certain hydrophobicity
Microdomains - 50% of proteins in cell membrane
Cell Membrane Microdomains
Examples: 1. Lipid Raft
- Thickened portion of the lipid bilayer comprising
about 50% and rich in cholesterol and sphingolipids
- Rich in sphingolipids and cholesterol where the GPI-anchoring protein can be seen
- Important in anchorage and cell-cell interaction and signaling
2. Caveola
- Curved lipid raft, due to the action of caveolin, a protein which dimerizes with other caveolin through its fatty acyl and cholesterol moities, producing a strain and causing the cell membrane to invaginate.
- Important in various cellular process involving membrane breakage and sealing, and signal transduction.
Endocytosis and Exocytosis
For bigger substances to transport a. Endocytosis - the entrance of substances, smaller
macromolecules, and other debris through a cell membrane mediated engulfment without leakage of
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 10 of 16
cell contents or without the loss of cellular membrane integrity
b. Exocytosis - the release of substances, smaller
macromolecules,and other substances for transport to othe issues and cells through a celmembrane mediated release without leakage of cell contents or without the loss of cellular membrane integrity
Cell Moembrane Transport
Transfer of solutes and information across membranes 1. Diffusion 2. Simple diffusion (passive and facilitated) 3. Cross-membrane movement of large molecules 4. Endocytosis 5. Exocytosis
• Signal transmission across membrane
Cell surface receptors 1) Signal transduction (glucagon cAMP) 2) Signal internalization (coupled with endocytosis like
LDL receptos, Insulin and Glut transporters)
Movement to intracellular receptors (steroid hormones, thyroid hormones, retinoids, Vit. D – hydrophobic in nature so they do not need transport proteins; easily pass through the membrane)
Creation of Electrochemical Gradient
Depends on the chemical gradient (or the difference of solute concentration or its ratio C2/C1) and the transmembrane electrical gradient, Vm in millivolts. Solutes follow the 2nd law of thermodynamic where it tends to assume spontaneously the greatest randomness and the lowest energy
Diffusion
Simple diffusion without membrane protein
- movement of solutes down its electrochemical gradient due random thermal movement or simply because the solute is permeable through the lipid bilayer because it is small enough and/or hydrophobic
Facilitated diffusion
- movement of solutes down its electrochemical gradient through either transporters or ion channels (membrane proteins)
How do transporters allow the passage of hydrophobic
solute? - For proteins to transverse the lipid bilayer, they have to
interact with the hydrophobic core within the chain of the sequence of amino acids protrudes out the hydrophilic part, e.g. the hydrogen and nitrogen, making the core ionic in nature so hydrophilic solute can pass through
- By lowering the energy of activation of the solute (ex. hydrated Na
+ ion needs much energy to pass
through the membrane but transporters lower the free energy change)
Passive and Active Transport
Free diffusion – H2, CO2, urea, O2 Facilitated diffusion –downhill but does not need energy
Comparison between Transporters and Ion channels
1. Transporter- alternating gates - Binds molecule and ion with high specificity - Undergoes conformational change to let the transported
ion enter the other side of the membrane - Saturable/ acts like an enzyme - Involves active and passive transport (facilitated
diffusion) 2. Ion Channels – single gate
- Show some specificity for the solute but does not act like an enzyme and only forms pores which open and close with less conformational change
- Non-saturable - Involve mostly facilitated diffusion
Uniport, Symort, Antiport
A. Glucose - through passive transport and a uniport which is used in transporting glucose in the cell through the influence of insulin
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 11 of 16
B. Glucose - may also be transported together with sodium into cell as a symport type of facilitated transport
C. Chloride-bicarbonate exchanger of the RBC membrane - allows the transport of bicarbonate without changing the membrane potential in opposite direction with chloride ions and increases the carbon dioxide carrying capacity of blood
Aquaporins
Water channels that only allow the passage of water molecules in the membranes of RBC and cells of the collecting ductules of the kidney
Mutation in some of these channel proteins may cause Nephrogenic Diabetes Insipidus [a disorder in which a
defect in the small tubes (tubules) in the kidneys causes a person to pass a large amount of urine].
Comparison between Ionotropic Channels and Ionophores
Ionotropic Channels Ionophores
Receptors that acts as channels and do not need a secondary messenger
Non-receptor compounds that produce channels or pores in the cell. If it produces pores or channels in bacteria they serve as anti-bacterial. While if they cause production of pores or channels on cell membranes of human cells, they cause injury or disease.
a. Acetylcholine- Na+ and Ca2+
b. Serotonin and Glutamate-K+, Na+, Ca2+
c. Glycine-Cl- specific
channels
Valinomycin-K+ channels Monensin-Na+ channels Gramicidin-folding creates hollow channels
a. Dendrotoxin (mamba snake)- K
+
b. Tetrodotoxin (puffer fish)- Na+
c. Cobrotoxin and alpha conotoxin-
d. Acetylcholine receptor ion channel
Diptheria toxin and activated complement- create large cellular pores causing lysis Alpha-hemolysis (Strep.), leak out ATP
TERMS
Active transport - the diffusion of molecules and ions against its
electrochemical gradient with the use of energy which is mostly supplied by ATP; its protein transporter has an ATPase activity hydrolyzing ATP to ADP producing the needed energy.
Primary Active Transport - the solute accumulation coupled
directly to an exergonic chemical reaction such as breakdown of ATP
Secondary Active transport- occurs when endergonic (uphill)
transport of one solute is coupled to an exergonic (downhill) flow of a different solute that was originally pumped uphill a primary active transport
Diagram (directly) above summarizes cell membrane transport mechanisms.
Importance of Ion Channels
Venom of animals like snakes targets the ion channels instead of proteins of metabolism. If it wanted to take down another organism, it would inhibit the other’s glycolytic pathway. Ion channels and the metabolic pathway are both composed of proteins (enzymes n the metabolic pathway). *In science the straight forward line going to
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 12 of 16
the answer is the easiest and most correct one*. In the evolutionary sense it is always through the conservation that our species (or other species) perpetuate. Animals with poisons want to make their venom more effective by targeting ion channels since even when targeting those alone the venom will already affect numerous parts of the body. In the case of action potentials, when these are disturbed/interrupted an animal/human may be paralyzed right away. Paralysis due to targeting proteins of the glycolytic pathway may take more time compared to the immediate paralysis by disruption of ion channels.
Cell Membrane Transport
Some types of ATP-deriven Active Transporters
Type Example Cellular Location
P-Type
Ca2+
ATPase Sarcoplasmic
Reticulum
N+-K
+ ATPase
Plasma or cell membrane
F-Type
mt ATPase synthase of
oxidative phosphorylation
Mitochondria
V-Type
The ATPase that pumps protons into lysosomes and synaptic
vesicles
Lysosomal and synaptic vesicular
membranes
ABC Transported
CFTR protein Plasma or cell
membrane
MDR I protein Plasma or cell
membrane
Some of the types of ATPase (from Harper) are listed on the table above. The most important to remember is your sarcoplasmic reticulum, a specialized form of endoplasmic reticulum in the myocyte. This facilitates occurrence of muscle contraction.
Ion Channels and Electrical Signals in Excitable Cells
The membranes of nerve cells contain well-studied ion channels
that are responsible for the generation of action potentials. The
activity of these channels is controlled by neurotransmitters; hence,
channel activity can be regulated (Murray, R.K., Bender, K.M.,
Kennelly, P.J., Rodwell, V.W., Weil, P.A., 2012).
Ion channels are open transiently and thus are “gated”. Gates can
be controlled by opening or closing. In ligand-gated channels, a
specifc molecule binds to a receptor and opens the channel.
Voltage-gated channels open (or close) in response to changes in
membrane potential. Mechanically-gated channels respond to
mechanical stimuli like pressure or touch (Murray, R.K., Bender,
K.M., Kennelly, P.J., Rodwell, V.W., Weil, P.A., 2012).
Characteristics of ion channels are listed in Tables 40-4 and 40-5
of Harper.
An action potential is one that causes the repolarization of the cell
membrane of the axon which may lead to activation of post-
synaptic vesicles of the post-synaptic neurons, or the contraction
of muscles which is brought about by the ionic composition within
the cell and the external environment. In the following Table 40-1,
we find that the extracellular fluid is very high in sodium (140
mmol/L) however the intracellular fluid only has 10 mmol/L of Na+.
The potassium (K+) on the other hand is very high in the
intracellular fluid (140 mmol/L) while very low (only 4 mmol/L) in
extracellular fluid. There is a discrepancy between sodium and
potassium levels. Mataas ang sodium sa labas, mababa sa loob;
mataas naman ang potassium sa loob, mababa sa baba.
Similarly so, Calcium is like Sodium and Chloride because they
are depolarizing ions. Remember this, the sodium, calcium, and
chloride ions are depolarizing.
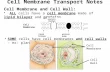
Dr. Hilario introduced the ion-gated channels just to give us the principle behind them (but this would be discussed in more detail in physiology). And asked the question: What kind of transport is this (Sodium-potassium ATPase)? Anti-core, primary active transport! For the action of this transporter, for every three potassium ions out, enters two sodium ions (see schematic diagram that follows).
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 13 of 16
There is an imbalance: there is negativity inside the cytoplasm and positivity outside it. This is the membrane potential which is about
-
50 to -70 mV. So any perturbation in the cell membrane like
pinching or any injury or stimulation will cause the leakage of sodium through the sodium channels where it goes inside causing depolarization of the membrane (Ano yung tatlong ion na nag-de-depolarize? Those are sodium, chloride, and calcium ions). So this time sodium depolarizes the membrane. It goes through the membrane until it reaches the neuromuscular junction. It will then depolarize the vesicle that releases the Acetylcholine (ACh) which will then attach to the receptor in the post-synaptic axon. Depolarization of sodium will continue until it reaches the neuromuscular junction and release the calcium through the T-tubule so that it propagates the action potential. The neuron starts the action potential. It’s the same membrane in
the diagrammatic scheme (see second to the last page). It goes to
the neuromuscular junction and the muscle contracts causing the
movement. If you look at the very small part of the axon, it would
reach the neuromuscular junction and the neuron will act on the
skeletal muscle.
You can see the release of the neurotransmitter, ACh in the diagram, so that it would act on the post-synaptic vesicles. Post-synaptic vesicles will then continue the action potential wherein sodium depolarizes the cell membrane. The acetylcholine binds to the receptor of the motor end plate of the neuromuscular junction. This depolarizes the cell membrane of the muscle fiber and going to the tubules releasing the calcium from the sarcoplasmic reticulum. The T-tubules are invaginations of the cell membrane. The same sodium channel acts on the membrane. It then releases the calcium. When there’s calcium it will stimulate the troponin until it works its way through actin-myosin binding so that there is movement. The sarcomere shortens thus causing the contraction. It moves along until it provides you the movement. Sodium is the one that leads the action potential. Chloride and calcium also depolarizes while potassium hyperpolarizes. This diagram presents Ach as a receptor in a ligand-gated channel (two images in the last page). The others formerly presented are ion-gated channels.
DISEASES INVOLVING ABNORMALITIES OF MEMBRANES
Table 40-7 from Harper’s lists diseases involving abnormalities in
Cell Membranes. Those enclosed in red rectangles are the ones
emphasized by Dr. Hilario.
Problem with cystic fibrosis: secretions are thick because there’s a mutation in the gene encoding the CFTR protein which is a chloride transporter. When chloride passes through the ion channel what comes with it? Sodium does. Water follows sodium. Water hydrates the lining of the respiratory membrane. Without water, the lining becomes thick; bacteria tend to be trapped in it. Most of cystic fibrosis patients die of pneumonia.
DISEASES INVOLVING ION CHANNEL DEFECTS
Table 11-7 below lifted from Dr. Hilario’s lecture notes. Note
emphasized items.
membrane
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 14 of 16
For the ion channel defects, we have the sodium-gated channel for
hyperkalemic periodic paralysis (Dr. Hilario doesn’t want us to
know the clinical part here. Just associate it with ion channels so
that you can at least know the relevance of studying these
diseases in relation to the ion channels). Ion channels defects of
skeletal muscle responsible for the clinical entity: hyperkalemic
periodic paralysis.
ARTIFICIAL MEMBRANES AND THEIR IMPORTANCE
When you look at the solution of aliphatic hydrocarbons in solution
with aqueous membranes you will find the electron micrograph like
this (as in image above). Each layer is a bilipid layer. When you
subject this to ultrasound frequency but mostly sonication, it
creates a vesicle-like structure where there is an aqueous cavity
inside. But still it is bounded by the lipid bilayer.
IMPORTANCE OF ARTIFICIAL MEMBRANES
1. Drug delivery where liposome, being hydrophobic in nature,
can easily enter the normal cell, cancer cell, and bacterial cell
So anything that can be dissolved in aqueous solution (that
you can put inside the cavity) can be transported through the
membrane so easily. Anti-cancer drugs, which are usually
hard to transport through the membrane because of their ionic
character, can be easily transported by liposome. The
importance of liposome is mainly (at the moment) for drug
delivery. Liposome being hydrophobic in nature can easily
enter the cell, i.e. cancer cells and bacterial cells. There are
liposomal preparations of doxorubicin which is a neomycin
that is anthracite-based chemotherapeutic agent for most
solid tumors, and cancer. There is also liposomal preparation
of ticarcillin and tobramycin which are used on most resistant
bacteria that resist usual preparations of those antibiotics.
With the presence of liposomal carriers they can easily
penetrate the membrane and gain access to bacteria in order
to facilitate bacterial destruction.
2. Gene therapy – liposome can easily penetrate the nucleus
that gene insert may be easily incorporated.
Gene therapy has failed before since most vectors used are
HIV viruses (retroviruses). E ‘diba natatakot ang tao, pag
retrovirus HIV yun. So how could you prevent infection with
HIV if the vectors for gene therapy are HIV viruses? So the
first experiment for gene therapy of ADA (adenosine
deamylase) for severe combined immune deficiency disease
was cancelled.
In the future, liposomal insertion (gene insert) into the
chromosome through the nucleoplasm through the nuclear
membrane may be easily achieved through liposomal carriers.
3. The Phospholipid (PL) may be attached to an antibody where
drug delivery may be target-oriented.
Anything may be attached to phospholipids which are ionic
parts of the cell membrane. You can attach protein which
attach to antibodies so you can target whatever you want to
target (most especially cancer cells).
4. Studies and researches on the activity and property of various
lipid and protein components of the cell membrane will soon
be made possible.
References:
Dr. Allan Hilario Lecture and Notes
Murray, R.K., Bender,K.M., Kennelly, P.J., Rodwell, V.W.,
Weil, P.A. (2012). Harper’s Illustrated Biochemistry (29th
ed.). North America: McGraw Hill Companie, Inc.
Images:
Dr. Allan Hilario Lecture and Notes
http://www.zoology.ubc.ca/~gardner/synapses%20-
%20presynaptic.htm
http://www.cnsforum.com/imagebank/item/rcpt_sys_nic_ag1/default.aspx
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 15 of 16
Metabolic Terms
Definition Function Cellular Location
Glycolysis
Breakdown of glucose and other hexoses to pyruvate or lactate
Production of ATP for energy need of the ell and for lipogenesis
Cytoplasm of all cells
Gluconeogenesis Synthesis of glucose from glucogenic precursor
Use of glucose to undergo glycolysis for energy need in case of scarce food source
Cytoplasm of hepatocytes and renal cells; and in special conditions intestinal cells
Hexose Monophosphate Shunt
Breakdown of glucose and other hexoses for the production of reducing equivalent and the sugar ribose
Provide the reducing equivalents for most synthetic pathways and the sugar backbone (ribose) for DNA and RNA synthesis
Cytoplasm of all cells
Glycogenolysis Breakdown of glycogen into glucose
To provide glucose readily from its storage form (glycogen) for energy need
Cytoplasm of hepatocytes and muscle cells
Glycogenesis Synthesis of glycogen from glucose
To store excess glucose from the diet in glycogen form
Cytoplasm of hepatocytes and muscle cells
Tricarboxylic Acid Cycle or TCA or Kreb’s Cycle
Central metabolic pathway of all cells
To burn the acetyl-CoA made from fat, glucose, or CHON to make ATP in cooperation with oxidative phosphorylation
Mitochondria of all cells (partly cytoplasm)
Oxidative phosphorylation and the electron transport chain
Pathway for producing the ATPs from all electron contributing pathways for energy needs
To synthesize ATPs from electrons from NADH and FADH
Mitochondria of all cells
Lipolysis (Beta-Oxidation)
Degradation of fatty acids for energy need
To break down fatty acids from acetyl-CoA
Mitochondria of all cells
Lipogenesis Synthesis of lipids To synthesize fatty acids from acetyl-CoA
Fat cells and Part of it in the cytoplasm
Alpha and Beta-Oxidation
Same as Beta-Oxidation
To breakdown FA with more than 18 carbons
Peroxisomes
Omega-oxidation Same as Beta-oxidation
To breakdown medium and long-chain fatty acids
Peroxisomes
Replication Synthesis of DNA To provide duplicate copies of the DNA for cell division
Nucleus
Transcription Synthesis of RNA
To serve as a template of nformation transfer from the DNA of the genes and for protein synthesis and the other types of RNA
Nucleus
Translation Synthesis of proteins To synthesize protein gene expression
(rER) cytoplasm
Post-translational modification
Modification of protein molecules after its synthesis
To provide the protein molecules its mature function
Multiple
Protein Sorting Packaging of protein either for temporary storage or transport
To direct the release of protein to its target of action
Golgi apparatus
Ketogenesis Excess acetyl-CoA is converted to ketone bodies
To provide fuel source especially the brain during prolonged starvation
Matrix of mitochondria of hepatocytes (in cytoplasm); and in special conditions renal cells
Urea Cycle
Cyclical pathway that produces urea from ammonia,CO2 and aspartate
To dispose approximately 90% of surplus nitrogen in the presence of excess amino acids as in excess intake or starvation
Hepatocytes
BIOCHEMISTRY // CELL AND CELL MEMBRANE
Page 16 of 16
Metabolic Terms
Definition Function Cell Location
Amino Acid Synthesis
The synthesis of amino acids
To provide the amino acids needed for protein synthesis
rER (Cytoplasm) Paanong RBC? Once nucleated precursors of RBC are already removed of cytoplasm, the RBC is already complete, it’s just waiting itself to die after 120 days. Only function is to transport haemoglobin.
Amino Acid Catabolism
Degradation of amino acids
To provide intermediates for energy needs and as dependent compounds
Cytoplasm of all cells
Volatage-gated Channel
Ligand-gated Channel
Ligand-gated Channel
*Illustration lifted from Dr. Hilario’s Lecture Notes*
Related Documents