09-Aug-2008-JAN-2007-BE-1263-SS Adapted from Buse JB et al. In Williams Textbook of Endocrinology. 10th ed. Philadelphia, Saunders, 2003:1427–1483; Buchanan TA Clin Ther 2003;25(suppl B):B32–B46; Powers AC. In: Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill, 2005:2152–2180; Rhodes CJ Science 2005;307:380–384. The Pathophysiology of Type 2 Diabetes Includes Three Main Defects Hyperglycemia Liver Insulin deficiency Excess glucose output Insulin resistance (decreased glucose uptake) Pancreas Muscle and fat Excess glucagon Islet Diminished insulin Diminishe d insulin Alpha cell produce s excess glucago n Beta cell produces less insulin Sulfonylurea ; Novonorm Metformin glitazonen

09-Aug-2008-JAN-2007-BE-1263-SS Adapted from Buse JB et al. In Williams Textbook of Endocrinology. 10th ed. Philadelphia, Saunders, 2003:1427–1483; Buchanan.
May 13, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Adapted from Buse JB et al. In Williams Textbook of Endocrinology. 10th ed. Philadelphia, Saunders, 2003:1427–1483; Buchanan TA Clin Ther 2003;25(suppl B):B32–B46; Powers AC. In: Harrison’s Principles of Internal Medicine. 16th ed. New York: McGraw-Hill, 2005:2152–2180; Rhodes CJ Science 2005;307:380–384.
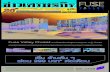
The Pathophysiology of Type 2 Diabetes Includes Three Main
Defects
Hyperglycemia
Liver
Insulin deficiency
Excess glucose output
Insulin resistance (decreased glucose
uptake)
Pancreas
Muscle and fat
Excess glucagon
Islet
Diminishedinsulin
Diminishedinsulin
Alpha cellproduces excess glucagon
Beta cellproduces less insulin
Sulfonylurea ; Novonorm
Metformin
glitazonen

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Adapted from Brubaker PL, Drucker DJ Endocrinology 2004;145:2653–2659; Zander M et al Lancet 2002;359:824–830; Ahrén B Curr Diab Rep 2003;3:365–372; Buse JB et al. In Williams Textbook of Endocrinology. 10th ed. Philadelphia, Saunders, 2003:1427–1483.
Incretins Regulate Glucose Homeostasis Through Effects on
Islet Cell Function
Active GLP-1 and
GIP
Release of incretin gut hormones
Pancreas
Bloodglucose control
GI tract
Glucagon from alpha cells
(GLP-1)Glucose
dependent
Alpha cells
Increased insulin and decreasedglucagon reduce hepatic glucose output
Glucose dependent Insulin
from beta cells(GLP-1 and GIP)
Beta cells
Insulinincreases peripheral glucose uptake
Ingestion of food

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
GLP-1 and GIP zijn de twee belangrijkste Incretines
GLP-1Glucagon-like peptide 1
GIPGlucose-dependent
insulinotropic polypeptide • Secretie door L-cellen in
distale darm (ileum en colon)• Stimuleert glucose-
dependente insulinevrijzetting
• Secretied door K-cellen in de proximale darm (duodenum)
• Stimulateert glucose-dependente insulinevrijzetting
• Suppressie van de hepatische glucoseproduktie door inhibitie van de glucagon secretie
(effect op adipocyten)
• verhoogt beta-cel proliferatie en overleving in diermodellen en geisoleerde humane eilandjes
• verhoogt beta-celproliferatie en overleving in eiladjes cellijnen
GLP-1=glucagon-like peptide 1; GIP=glucose-dependent insulinotropic polypeptideAdapted from Drucker DJ Diabetes Care 2003;26:2929–2940; Ahrén B Curr Diab Rep 2003;3:365–372; Drucker DJ Gastroenterology 2002;122:531–544; Farilla L et al Endocrinology 2003;144:5149–5158; Trümper A et al Mol Endocrinol 2001;15:1559–1570; Trümper A et al J Endocrinol 2002;174:233–246.

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Effect van 6 weken behandeling met GLP-1 infuus
bij patiënten met type 2 diabetes
• Verlaging van nuchtere glycemie met 77 mg/dl en gemiddelde glycemie met 100 mg/dl
• Verlaging van HbA1c met 1,3 %• Gewichtsdaling met 2-3 kg• Verbetering van de insulinegevoeligheid met 77 %
Snelle inactivatie (enzyme DPP-4),
Korte eliminatie : t 1/2 ~1-2 min
GLP-1 moet via continu infuus toegediend worden
Ongeschikt voor behandeling van een chronische ziekte zoals type 2 diabetes
Drucker DJ, et al. Diabetes Care. 2003;26:2929-2940.

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Strategieën voor Verbetering van het Therapeutisch Potentieel van GLP-1
• Produkten die de werking van GLP-1 nabootsen (incretin mimetics)–DPP-4–resistente GLP-1 derivaten
•bv: GLP-1 analogen, albuminegebonden GLP-1
–Nieuwe peptiden met glucoseregulerende werking gelijkaardig aan GLP-1
•Exenatide
• Produkten die de activiteit van endogeen GLP-1 verlengen (incretin enhancers)–DPP-4 inhibitors
•Bv sitagliptine (Januvia, Merck), vildagliptine (Galvus, Novartis), SYR 322 (Takeda, fase 3 studies)
Drucker DJ, et al. Diabetes Care. 2003;26:2929-2940.; Baggio LL, et al. Diabetes. 2004;53:2492-2500.

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Hb
A1
c (
% ±
SE
)
LS mean change from baseline (for both groups): –0.67%
Achieved primary hypothesis of
noninferiority to sulfonylurea
aSpecifically glipizide; bSitagliptin (100 mg/day) with metformin (≥1500 mg/day); Per-protocol population; LS = least squares
Adapted from Nauck et al. Diabetes Obes Metab. 2007;9:194–205.
52-week Sitagliptin vs Sulfonylureaa Add-on Therapy to Metformin Study
Sitagliptin Once Daily Showed Comparable Glycemic Efficacy to Sulfonylurea When Added to Metformin (52
Weeks)
Weeks
5.8
6.0
6.2
6.4
6.6
6.8
7.0
7.2
7.4
7.6
7.8
0 6 12 18 24 30 38 46 52
Sulfonylureaa + metformin (n=411)
Sitagliptinb + metformin (n=382)

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
-3
-2
-1
0
1
2
3
0 12 24 38 52
Weeks
52-week Sitagliptin vs Sulfonylureaa Add-on Therapy to Metformin Study Sitagliptin Provided Weight Reduction (vs Weight Gain) and a Much
Lower Incidence of Hypoglycemia
Sulfonylurea + metformin (n=584)Sitagliptin 100 mg/day + metformin (n=588)
Hypoglycemiab
P<0.001
32%
5%
0
10
20
30
40
50
Week 52In
cide
nce
(%)
LS mean change in body weight over timeb
Bod
y w
eigh
t (k
g ±
SE
)
aSpecifically glipizide; bAll-patients-as-treated population. LS = least squares; LSM between-group difference at week 52 (95% CI): in body weight = –2.5 kg [–3.1, –2.0] (P<0.001);LSM change from baseline at week 52: glipizide: +1.1 kg; sitagliptin: –1.5 kg (P<0.001)Adapted from Nauck et al. Diabetes Obes Metab. 2007;9:194–205.
Sulfonylurea + metformin (n=416)
Sitagliptin 100 mg/day + metformin (n=389)

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Strategieën voor Verbetering van het Therapeutisch Potentieel van GLP-1
• Produkten die de werking van GLP-1 nabootsen (incretin mimetics)–DPP-4–resistente GLP-1 derivaten
•bv: GLP-1 analogen, albuminegebonden GLP-1
–Nieuwe peptiden met glucoseregulerende werking gelijkaardig aan GLP-1
•Exenatide
• Produkten die de activiteit van endogeen GLP-1 verlengen (incretin enhancers)–DPP-4 inhibitors
•Bv sitagliptine (Januvia, Merck), vildagliptine (Galvus, Novartis), SYR 322 (Takeda, fase 3 studies)
Drucker DJ, et al. Diabetes Care. 2003;26:2929-2940.; Baggio LL, et al. Diabetes. 2004;53:2492-2500.

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Exenatide (Exendin-4)
– Synthetic version of salivary protein found in the Gila monster
– Approximately 50% identity with human GLP-1
– Resistant to DPP-4 inactivation
Development of Exenatide: An Incretin Mimetic
Adapted from Nielsen LL, et al. Regulatory Peptides. 2004;117:77-88. Reprinted from Regulatory Peptides, 117, Nielsen LL, et al, Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycaemic control of type 2 diabetes, 77-88, 2004, with permission from Elsevier for English use only.
Site of DPP-4 Inactivation
H G E G T F T S D L S K Q M E E E A V R L F I E W L K N G G P S S G A P P P S – NH2
H A E G T F T S D V S S Y L E G Q A A K E F I A W L V K G R – NH2
Exenatide
GLP-1Human

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
-0.5
-1.5
-1
0
-0.9 *
-0.6 *
+0.1
-0.7
-1.4 *
-1.9 *
-2.0
-1.5
-1.0
-0.5
0
Large Phase 3 Clinical Studies: Exenatide bid Reduced HbA1c and
Weight Over 30 Weeks
ITT 30-week data; N = 1446; Mean (SE); *p<0.005; Weight was a secondary endpoint.Data on file, Amylin Pharmaceuticals, Inc. * DeFronzo RA, et al. Diabetes Care. 2005;28:1092-1100.; Buse JB, et al. Diabetes Care. 2004;27:2628-2635.;Kendall DM, et al. Diabetes Care. 2005;28:1083-1091
Ch
ang
e in
Hb
A1c
(%)
Ch
ang
e in
Wei
gh
t (k
g)
Placebo BID Exenatide 5 µg BID Exenatide 10 µg BID
Combined Results of 3 Exenatide Phase 3 , Placebo-controlled Studies*

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Lange termijn effecten van Byetta op HbA1c en gewicht
0 10 20 30 40 50 60 70 80 90 1001106.5
7.0
7.5
8.0
8.5
8.3%
-1.1%
0 10 20 30 40 50 60 70 80 90 100110-7
-6
-5
-4
-3
-2
-1
0
1
100 kg
-4.7 kg

09
-Au
g-2
00
8-J
AN
-20
07
-BE-1
26
3-S
S
Waar situeren ?
• Gliptines in 2de lijn na metformin– Gewicht– Geen risico op hypo’s– Weinig neveneffecten “instapmodel”– Af : HbA1c > 7 % onder metformine– Starten met staal !
• GLP-1 analogen vóór insuline– Gewicht– Minder risico op hypo’s– Cave misselijkheid– Af : HbA1c > 7,5 % onder metformine + sulfonylureum
• Drempel vooral financieel en Af
Related Documents