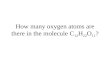e water molecule two hydrogen and one oxygen H20

The water molecule two hydrogen and one oxygen H20.
Mar 27, 2015
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

The water molecule two hydrogen and one oxygen H20

The shape of the water molecule results in it forming the familiar six sided snowflake structure. Also when water freezes the molecules arrange themselves as shown above leaving empty space within the six-sided ring structure. This is why 1.0 gram of ice is larger in volume than 1.0 gram of liquid water.

Water molecules continually evaporate from and condense back onto the liquid water surface. If the evaporation rate is larger than condensation rate the humidity increases and if the evaporation rate is smaller than the condensation rate then the humidity decreases. The air is said to be saturated when the evaporation and condensation rates are equal.

The air is said to be saturated when the evaporation and condensation rates are equal. In a closed system the saturation humidity increases as the temperature increases. The reason for this is that the evaporation rates increase and hence the number of water vapor molecules the air increases until the condensation rate matches this higher evaporation rate.



http://ga.water.usgs.gov/edu/watercycle.html
Water Cycle: See hydrological Cycle Module

Figure 2. The mean distribution of precipitable water, or total atmospheric water vapor above the Earth's surface, for 1992. This depiction includes data from both satellite and radiosondeobservations. (Image courtesy of Thomas Vonder Haar and David Randel, Colorado State University, Fort Collins.) On average over the whole Earth there is about 1 inch (25 mm) of precipitable water in the atmosphere at any given time. This is equivalent to about 1 week’s worth of water use by humans.

There is typically more water near the surface than higher up. The stratosphere is quite dry.

On average the near surface equatorial regions (the tropics) have the largest humidity of anywhere on Earth.

Water vapor is extremely variable from place to place and time to time. Water vapor amounts recorded from Satellites can help forecasters determine heavy precipitation events and also help estimate nighttime lows from radiative cooling.

Since water molecules emit infrared radiation IR detectors can be used to sense water vapor amounts remotely.

Since water vapor is a greenhouse gas it can trap heat energy absorbed by the sun close to the surface throughout the night. Les water vapor implies cooler night-time temperatures. The difference between day time and night time temperatures is greatest in desert regions that have little water vapor.

In any given parcel of air there are nitrogen oxygen, and water molecules (water vapor) among many other gases in small quantities. The actual humidity is a measure of how many water molecules are actually in the air. The actual humidity can be measured in a variety of units. Grams of water per kilogram of air or grams of water per cubic meter.
We will use vapor pressure as a measure of humidity. As the molecules bounce around wildly they exert an outward pressure. At sea level this outward pressure balance the inward pressure from the weight of all air above a location. This is normal sea level pressure.

We will use vapor pressure as a measure of humidity. As the molecules bounce around wildly they exert an outward pressure. At sea level, this outward pressure balances the inward pressure from the weight of all air above a location. This is normal sea level pressure and is about 1000 mb (milli-bars). Part of this outward pressure comes from the water vapor molecules.
When the vapor pressure is 20 mb approximately 2% of all air molecules are water vapor. When the vapor pressure is 10 mb approximately 1% of all air molecules are water vapor. When the vapor pressure is 30 mb approximately 3% of all air molecules are water vapor.

Water boils when the internal vapor pressure pushing out on a tiny bubble equals the atmospheric pressure pushing inward on the bubble. The graph above shows the saturation vapor pressure on the y-axis versus temperature on the x-axis. Water normally boils at 100 oC at sea level because at that temperature the saturation vapor pressure equals the sea level pressure.

Saturation Vapor Pressure vs Temperature
500
600
700
800
900
1000
80 90 100 110
Temperature (°C)
Mt. Hood
Mt. Rainer
At the top of Mt. Hood water boils at about 93 oC
At the top of Mt. Rainer water boils at about 85 oC

Amazingly Vancouver Wa and Tucson Az have the same amount of water vapor in the air during January.

Dew point Temperature(directly linked to actual humidity)
Temperature that results in saturation or dew forming on surfaces.
High dew point high actual humidityLow dew point low actual humidity
Dew points in excess of 70 F will normally make one uncomfortable.

The dew point temperature is directly linked to actual humidity

Vancouver Wa and Tucson Az also have roughly the same amount of water vapor in the air during July.

Relative Humidity
RH Actual _ Humidity
Saturation _Humidity

Saturation Vapor Pressure (mb) vs Temp. C
0
5
10
15
20
25
30
35
40
-10 0 10 20 30
Temperature (°C)
A
B
The relative humidity describes how much water is in the atmosphere relative to the maximum possible water vapor amount. In the above figure the dark blue columns indicate the vapor pressure (actual) and the total column height up to the top of the light blue is the maximum possible (saturation vapor pressure). Graphically we can see that the far right column has the highest relative humidity and the middle has the lowest.

High relative humidity results in low evaporation rates so our body does not cool itself very well and we more easily suffer from heat stroke. The Heat index is a quantitative measure of this discomfort. The above example shows an air temperature of 100 oF with relative humidity of 60% is equivalent to a dry 130 oF day. During most years, heat stroke is the number 1 weather related cause of death in the US.

These examples of cooling from evaporation work best in dry climates.
Evaporative cooler, porous clay pot, and canvas canteen

If you soak the canvas wrapper the evaporative cooling will keep you water cool.
However just wrapping your bottle in cloth with prevent condensation from occurring on the bottle. Since water vapor releases latent heat energy when it condenses, the cloth wrapping isolates you water bottle from warming up too fast from the condenstaion.

Fig. 4-11a, p. 93
The RH will increase if more water vapor is added to the air.

Fig. 4-11b, p. 93
Since as the air temperature increases so does the saturation vapor pressure. This causes the relative humidity decrease as the air temperature increases (denominator gets bigger)

RH is maximum when it is coolest. This is the most likely time for fog, dew or frost to occur. RH is minimum when it is warmest.

Outside
T = -15 CTd = -15 CRH =100%
Inside
T = 20 CTd = -15 CRH = 8%
Inside the cabin the air is uncomfortably dry.

Air temp= 35oCDew point = 10 oCRH =21 %
A dew point of 10 oC represents air with a significant amount of water. However since it is so warm here in this desert the relative humidity is quite low. Evapo-transpiration occurs quite rapidly from surface plants leaving the area relatively dry. This same dew point with a mean air temperature of 20 oC (68F) would correspond to a comfortable 52% relative humidity and plant life would likely flourish

Air coming off the Pacific is relatively cool and contains less water than the warm moist air flowing into the Eastern US from the Atlantic and Gulf of Mexico.

Hygrometers measure humidity
A Sling Psychrometer is common
We’ll use the sling psychrometer in our week 4 lab.

Computer data collection has almost completely replace the old style hydrographs.
Related Documents