Photocrosslinked nanocomposite hydrogels from PEG and silica nanospheres: Structural, mechanical and cell adhesion characteristics Akhilesh K. Gaharwar ⁎ , 1 , Christian Rivera, Chia-Jung Wu, Burke K. Chan, Gudrun Schmidt Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907-2032, USA abstract article info Article history: Received 31 August 2012 Received in revised form 5 October 2012 Accepted 31 December 2012 Available online 8 January 2013 Keywords: Photocrosslinked hydrogels Mechanical properties Nanocomposite Silica nanospheres Photopolymerized hydrogels are extensively investigated for various tissue engineering applications, primarily due to their ability to form hydrogels in a minimally invasive manner. Although photocrosslinkable hydrogels provide necessary biological and chemical characteristics to mimic cellular microenvironments, they often lack sufficient mechanical properties. Recently, nanocomposite approaches have demonstrated potential to overcome these defi- cits by reinforcing the hydrogel network with. In this study, we investigate some physical, chemical, and biological properties of photocrosslinked poly(ethylene glycol) (PEG)-silica hydrogels. The addition of silica nanospheres sig- nificantly suppresses the hydration degree of the PEG hydrogels, indicating surface interactions between the silica nanospheres and the polymer chains. No significant change in hydrogel microstructure or average pore size due to the addition of silica nanospheres was observed. However, addition of silica nanospheres significantly increases both the mechanical strength and the toughness of the hydrogel networks. The biological properties of these nanocomposite hydrogels were evaluated by seeding fibroblast cells on the hydrogel surface. While the PEG hydrogels showed minimum cell adhesion, spreading and proliferation, the addition of silica nanospheres enhanced initial cell adhesion, promoted cell spreading and increased the metabolic activity of the cells. Overall, results indi- cate that the addition of silica nanospheres improves the mechanical stiffness and cell adhesion properties of PEG hydrogels and can be used for biomedical applications that required controlled cell adhesion. © 2013 Published by Elsevier B.V. 1. Introduction Photopolymerization allows for the rapid and controllable synthesis of polymer networks in ambient physiological environments [1–3]. This method is extensively used in various biomedical applications, such as elastic tissue sealants for prevention of post-surgery adhesion [4–6], drug [7,8] and protein [9] delivery, and tissue engineering applications [10]. Pre-polymer solutions can be delivered in a liquid form and cross- linked by ultraviolet (UV) radiation in order to form an elastic hydrogel [11,12]. This gelation property not only provides the convenience of drug delivery, but also allows hydrogels to form inside the tissue using a minimally invasive procedure [11,12]. Several synthetic as well as natural polymers, such as poly(ethylene glycol) [13] (PEG), poly(vinyl alcohol) (PVA) [14], gelatin [15], hyaluronic acid [16,17], alginate [18], and chitosan [19], have been proposed to synthesize photocrosslinked hydrogels. Although these polymeric hydrogels provide some chemical and biological characteristics that mimic biological tissue, they lack sufficient mechanical properties [20,21]. Recently, nanocomposite approaches have shown potential in overcoming the drawbacks of a polymeric hydrogel network [22–27]. Polymer nanocomposites possess superior properties compared to macro- and micro-composites [28]. Nanocomposites offer several novel property combinations such as high toughness, elastomeric properties, gas barrier, and control release due to the molecular interaction of fillers with polymers at the nano scale [22]. Several types of inorganic nanoparticles, including (bioactive glasses, hydroxyapatite, β-tricalcium phosphate and silicate clay) have been used to enhance physical and bi- ological properties of polymeric hydrogels [29,30]. The nanoparticles can either be used as a cross-linker or as a filler entrapped within the hydrogel network [31]. For example, nanoparticles such as HA can be entrapped within the hydrogel network to increase the mechanical strength and bi- ological functionality of the polymeric matrices [32]. In another approach, silicate nanoplatelets (such as layered synthetic silicates) can be used as physical crosslinkers to form hydrogel networks [33–38]. In this study, nanoparticles not only improve the mechanical strengths of the poly- meric matrix but also impart various biofunctionalities that can be used for various biomedical and biotechnological applications. Recently, silica-based nanoparticles have been extensively investigat- ed for various biomedical and biotechnological applications such as ana- lytical tools [39,40], imaging [41,42], and controlled drug release and delivery [43,44]. This is mainly due to their unique optical properties, low density, high specific surface area, low toxicity and high adsorption capacity [45,46]. Materials Science and Engineering C 33 (2013) 1800–1807 ⁎ Corresponding author Tel.: +1 765 430 3069; fax: +1 765 496 1912. E-mail address: [email protected] (A.K. Gaharwar). 1 Current location: David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. 0928-4931/$ – see front matter © 2013 Published by Elsevier B.V. http://dx.doi.org/10.1016/j.msec.2012.12.099 Contents lists available at SciVerse ScienceDirect Materials Science and Engineering C journal homepage: www.elsevier.com/locate/msec

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
Materials Science and Engineering C 33 (2013) 1800–1807
Contents lists available at SciVerse ScienceDirect
Materials Science and Engineering C
j ourna l homepage: www.e lsev ie r .com/ locate /msec
Photocrosslinked nanocomposite hydrogels from PEG and silica nanospheres:Structural, mechanical and cell adhesion characteristics
Akhilesh K. Gaharwar ⁎,1, Christian Rivera, Chia-Jung Wu, Burke K. Chan, Gudrun SchmidtWeldon School of Biomedical Engineering, Purdue University, West Lafayette, IN 47907-2032, USA
⁎ Corresponding author Tel.: +1 765 430 3069; fax: +1E-mail address: [email protected] (A.K. Gaharw
1 Current location:DavidH. Koch Institute for IntegrativeInstitute of Technology, Cambridge, MA 02139, USA.
0928-4931/$ – see front matter © 2013 Published by Elhttp://dx.doi.org/10.1016/j.msec.2012.12.099
a b s t r a c t
a r t i c l e i n f oArticle history:Received 31 August 2012Received in revised form 5 October 2012Accepted 31 December 2012Available online 8 January 2013
Keywords:Photocrosslinked hydrogelsMechanical propertiesNanocompositeSilica nanospheres
Photopolymerized hydrogels are extensively investigated for various tissue engineering applications, primarily dueto their ability to form hydrogels in a minimally invasive manner. Although photocrosslinkable hydrogels providenecessary biological and chemical characteristics to mimic cellular microenvironments, they often lack sufficientmechanical properties. Recently, nanocomposite approaches have demonstrated potential to overcome these defi-cits by reinforcing the hydrogel network with. In this study, we investigate some physical, chemical, and biologicalproperties of photocrosslinked poly(ethylene glycol) (PEG)-silica hydrogels. The addition of silica nanospheres sig-nificantly suppresses the hydration degree of the PEG hydrogels, indicating surface interactions between the silicananospheres and the polymer chains. No significant change in hydrogel microstructure or average pore size dueto the addition of silica nanospheres was observed. However, addition of silica nanospheres significantly increasesboth the mechanical strength and the toughness of the hydrogel networks. The biological properties of thesenanocomposite hydrogels were evaluated by seeding fibroblast cells on the hydrogel surface. While the PEGhydrogels showedminimumcell adhesion, spreading and proliferation, the addition of silica nanospheres enhancedinitial cell adhesion, promoted cell spreading and increased the metabolic activity of the cells. Overall, results indi-cate that the addition of silica nanospheres improves the mechanical stiffness and cell adhesion properties of PEGhydrogels and can be used for biomedical applications that required controlled cell adhesion.
© 2013 Published by Elsevier B.V.
1. Introduction
Photopolymerization allows for the rapid and controllable synthesisof polymer networks in ambient physiological environments [1–3]. Thismethod is extensively used in various biomedical applications, such aselastic tissue sealants for prevention of post-surgery adhesion [4–6],drug [7,8] and protein [9] delivery, and tissue engineering applications[10]. Pre-polymer solutions can be delivered in a liquid form and cross-linked by ultraviolet (UV) radiation in order to form an elastic hydrogel[11,12]. This gelation property not only provides the convenience ofdrug delivery, but also allows hydrogels to form inside the tissue usinga minimally invasive procedure [11,12]. Several synthetic as well asnatural polymers, such as poly(ethylene glycol) [13] (PEG),poly(vinyl alcohol) (PVA) [14], gelatin [15], hyaluronic acid [16,17],alginate [18], and chitosan [19], have been proposed to synthesizephotocrosslinked hydrogels. Although these polymeric hydrogelsprovide some chemical and biological characteristics that mimicbiological tissue, they lack sufficient mechanical properties [20,21].
765 496 1912.ar).Cancer Research,Massachusetts
sevier B.V.
Recently, nanocomposite approaches have shown potential inovercoming the drawbacks of a polymeric hydrogel network [22–27].Polymer nanocomposites possess superior properties compared tomacro- and micro-composites [28]. Nanocomposites offer several novelproperty combinations such as high toughness, elastomeric properties,gas barrier, and control release due to the molecular interaction of fillerswith polymers at the nano scale [22]. Several types of inorganicnanoparticles, including (bioactive glasses, hydroxyapatite, β-tricalciumphosphate and silicate clay) have been used to enhance physical and bi-ological properties of polymeric hydrogels [29,30]. The nanoparticles caneither beused as a cross-linker or as afiller entrappedwithin the hydrogelnetwork [31]. For example, nanoparticles such as HA can be entrappedwithin the hydrogel network to increase the mechanical strength and bi-ological functionality of thepolymericmatrices [32]. In another approach,silicate nanoplatelets (such as layered synthetic silicates) can be used asphysical crosslinkers to form hydrogel networks [33–38]. In this study,nanoparticles not only improve the mechanical strengths of the poly-meric matrix but also impart various biofunctionalities that can beused for various biomedical and biotechnological applications.
Recently, silica-based nanoparticles have been extensively investigat-ed for various biomedical and biotechnological applications such as ana-lytical tools [39,40], imaging [41,42], and controlled drug release anddelivery [43,44]. This is mainly due to their unique optical properties,low density, high specific surface area, low toxicity and high adsorptioncapacity [45,46].
1801A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
In a recent study, the use of spherical silica nanoparticles to fabricatemechanically strong nanocomposite networks was also investigated[47]. Cai fabricated cellulose–silica nanocomposite by using the sol–gelmethod to obtain aerogels with high mechanical strength and flexibility,large surface area, and low thermal conductivity [47]. Yang introduced aversatilemethod to fabricate physically cross-linked hydrogels from silicananoparticles and poly(acrylic acid) [48]. They showed that physicalcross-linking between silica nanospheres and polymer leads to theformation of highly stable networks. In another approach, Takafujiused silica nanoparticles as multifunctional cross-linkers by mixingwith copolymer containing reactive side chains (trimethoxysilyl)[49]. The trimethoxysilyl side groups reacted with the hydroxylgroups present on the nanoparticles which resulted in the formationof cross-linked networks. Apart from reinforcing polymer networks,silica-based nanocomposites have also been proposed for tissueengineering applications. Cells readily attached on the surfaces ofsilica-hydroxyethylmethacrylate nanocomposites [50]. Moreover, somesilica nanoparticles are shown to be bioactive and can stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhancebone mineral density [51].
In this study, we developed photocrosslinkable PEG-silicananocomposites and evaluated the effect of silica on some physical,chemical, and biological properties of PEG hydrogels. We hypothesizedthat the addition of silica nanosphere to the PEG network enhancedme-chanical strength and provided the enhanced cell adhesion properties.The hybrid nanocomposite hydrogels have potential to be used in drugdelivery and biomaterials engineering applications.
2. Materials and methods
2.1. Materials
Polyethylene glycol (PEG) with a molecular weight Mw of12,000 g/mol (Dalton) was purchased from Fluka Analytical(Sigma-Aldrich). PEG diacrylate was synthesized according to a modifiedprocedure reported before [32,34,52]. Briefly, the hydroxyl groups of PEGwere acrylated by a five-fold molar excess of acryloyl chloride and afive-fold molar excess of triethylamine in 100 mL of dichloromethaneunder nitrogen. The reaction was stirred at room temperature for 24 h.The resulting solution was then filtered. Afterward PEG-diacrylate(PEGDA) was precipitated by pouring in cold diethyl ether. The whitePEG diacrylate precipitate was isolated and subsequently dried under avacuum for 1 day. The acrylation degree of PEGDA was >80%, as deter-mined by 400 Hz 1H NMR on a Bruker ARX 400 spectrometer and calcu-lated by the ratio of acryl protons of PEG diacrylate (CH2, δ=5.8–6.4)to \CH2O\ (δ=3.63) of PEG. Silica nanospheres (SiO2) with an av-erage size of 80 nm, a specific surface area of 440 m2/g and a specific sur-face density of 2.2–2.6 g/mL (25 °C)were purchased fromSigma-Aldrich.The photoinitiator, IRGACURE 2959, was purchased from Ciba AG (Basel,Switzerland).
2.2. Preparation of PEG-silica hydrogels
The nanocomposite hydrogels were prepared at ambient tempera-ture. First, the silica nanosphereswere dispersed in the initiator solution(0.2% IRGACURE 2959 dissolved in deionized water) by vortexing for10 min and sonicating for 20 min. Then, the PEG diacrylate was addedto the silica solution and the dispersionwas allowed tomix for addition-al 20 min. No segregation or phase separation after mixing, however ifsolution is allowed to stand for 1 h, silica particle start precipitating.The prepolymer solution was immediately injected into a moldand then photocrosslinked for 10 min by using a high-intensity UVlamp (B-100AP, Ultra-Violet Products, Upland, CA, wavelength used:365 nm). The distance between the sample and the UV lamp was keptat 15 mm. Different types of samples were prepared for the materialcharacterizations. For tensile testing, dumb-bell-shaped specimens
were prepared (5 mm in length, 3 mm wide, and 1 mm thick). Forthe rheological studies, cylindrical samples with a diameter of 20 mmand a height of 1 mm were used. For compression testing, cylindricalsamples with 5 mm diameter and 10 mm height were prepared.
2.3. Hydration kinetics
The hydration kinetics of the hydrogels were determined by usingcylindrical samples. First the samples were lyophilized for 24 h to re-move the entrappedwater and to preserve the porous structure of thedried hydrogel network. Then the initial weight of the samples wasdetermined. These samples were placed in distilled water at 4, 22,and 37 °C to determine the hydration kinetics. The weight of thehydrogels was determined at specific time points (0, 0.25, 0.75,1.25, 2, 3, 6, 8, 24, 48, 72, 96 h) and the swelling ratio was determinedby using: Swelling ratio=(Mo−Mt)×100/(Mo), where Mo is the ini-tial weight and Mt is the wet weight of the nanocomposite hydrogels.
2.4. Cryo-scanning electron microscopy (Cryo-SEM)
The microstructure of hydrogels was evaluated by using a FEI NOVAnanoSEM (Hillsboro, OR) using 5 kV accelerating voltage. First, hydrogelsamples were plunged in a liquid nitrogen slush, then transferred toGATAN Alto 2500 Cryo Units (Warrendale, PA) and cooled to −130 °C.The samples were fractured by using a cold scalpel; then, the fracturedsurfaces were sublimated at −90 °C for 8 min, followed by a sputtercoating with platinum at −130 °C for 90 s. Afterward, the sampleswere transferred to a cryo-stage (−140 °C) for imaging.
2.5. Mechanical properties
Mechanical properties of the hydrogels were determined by using anARES rheometer (TA Instruments) equipped with a 2000 gf transducer.Tensile properties of the hydrogels (n=5)weremeasured by subjectingthe sample to a strain rate of 5 mm/s. The elasticmoduluswas calculatedfrom the toe (0–50% strain) and linear regions (200–250% strain) of thestress–strain curve. The fractured stress and strainwere also determinedfrom the stress–strain curve. Compressive properties were determinedby subjecting the samples to a crosshead speed of 0.01 mm/s by usinga 20 mm parallel plate geometry at room termperature (22 °C). Hyster-esis (or energy lost due to permanent deformation) and recovery (in per-centage) was measured by subjecting the sample to a loading and anunloading cycle. Compressive modulus was calculated by determiningthe slope from the initial region (0–10% strain) of the stress–straincurve. Recovery was calculated by dividing the unloading curve by theloading curve.
2.6. In vitro cell studies
NIH-3T3 fibroblast cells (American Type Culture Collection)were cultured in Dulbecco's modified Eagle medium (DMEM, Gibco)supplemented with 10% bovine calf serum, 100 UmL−1 penicillin and100 μg−1 streptomycin and maintained in a 5% CO2 atmosphere at37 °C. Hydrogel films with different amounts of silica (0, 1, 5, and10 wt.%) were cut into 15 mm diameter circular discs and sterilizedby irradiating under high-intensity UV light for 2 h. Hydrogels wereswollen and fully hydrated in cell media before cell seeding. Cell surfaceadhesion was examined by seeding the hydrogel discs with cells at10,000 cells·cm−2. Cell media were changed every two days. Cellswere fixed with 3.7% formaldehyde in a phosphate buffer solution (PBS)solution and the cell cytoskeleton was labeled with an Alexa Fluor® 488phalloidin fluorescent dye (Invitrogen) at day 7 after seeding. Fluorescentimageswere obtained by using aNikon, Eclipse TE2000-S invertedmicro-scope. Cell adhesion and spreadingwere evaluated by using ImageJ (NIH)by determining the number of adhered cells per view and the average cellarea. MTS assays were performed following the manufacturer's protocol
Silica Nanoparticles
Poly(ethylene glycol) diacrylate
PI solution+
UV
1
2
34
5
a
b cComposition of PEG-Silica Nanocomposites
Samples Silica (%) PEG (%)
1 PEG 0 5
2 1% Silica 1 5
3 2.5% Silica 2.5 5
4 5% Silica 5 5
5 10% Silica 10 5
O
OO
O
Fig. 1. Synthesis of photocrosslinked PEG-Silica nanocomposite hydrogels. (a) Hydrogel solutions were prepared by mixing silica (x=0, 1, 2.5, 5, 10 wt.%) and PEG diacrylate (5%)in an initiator solution. The solutions were injected in a mold and cross-linked with UV light. (b) PEG hydrogels are transparent, but addition of silica nanospheres decreases theoptical transmittance. (c) Compositions of nanocomposite hydrogels.
1802 A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
for CellTiter 96® Aqueous One Solution Cell Proliferation Assay(Promega). Cells were seeded at 5000 cells·cm−2. The cell number foreach hydrogel sample was quantified with a SpectraMax M5 microplatereader to measure the absorbance at λ=490 nm.
2.7. Statistical analysis
Data are presented as mean (standard error of mean values).Statistical analysis was performed by using Minitab (version 16,
0 20 400
5
10
15
20
25
30
35
Sw
ellin
g R
atio
Time 0 20 40 60 80 100
0
5
10
15
20
25
30
35
Sw
ellin
g R
atio
Time (hours)
0 1 2.5 5 100
5
10
15
20
25
30
35
4 oC
22 oC
37 oC
Sw
ellin
g R
atio
Silica (%)
a
b
T = 4 oC
SilicaSilica
T =
Effect of Silic
T = 4 oC
n
0.1957
0.1750
0.1552
0.1240
0.0832
c
Fig. 2. Effect of silica nanospheres on hydration kinetics of PEG hydrogels. Hydration kinetidrogel readily hydrates and reaches an equilibrium hydration degree within 20 h. (b) PEG hdition of silica nanospheres. The addition of silica reinforces the network and restrict thenetworks were determined by fitting the initial hydration data. The characteristic swelling cport were calculated. Fickian diffusion was observed and addition of silica decreased the va
Minitab) to determine the statistical differences. Statistical compari-sons were performed with one-way analysis of variance (ANOVA) foran average of three to five replicates. After ANOVA was performed,Tukey's methodwas used to test all pairwisemean comparisons. Statis-tical significance for all tests was set to be at a p-valueb0.05.
3. Results and discussions
A prepolymer solution can be prepared by dissolving 5% acrylatedPEG in a photoinitiator solution containing 0.2% of IRGACURE 2959
60 80 100
(hours)0 20 40 60 80 100
0
5
10
15
20
25
30
350%1%2.5%5%10%
Sw
ellin
g R
atio
Time (hours)
Silica
22 oC T = 37 oC
a on Diffusion co-efficient of Nanocomposite Hydrogels
T = 22 oC T = 37 oC
k n k n k
3.1645 0.1679 3.2235 0.1487 2.976
2.9750 0.1462 3.1098 0.1284 2.9838
2.6925 0.1176 2.8686 0.1186 2.8471
2.6272 0.1029 2.7686 0.1107 2.7794
2.5918 0.0912 2.6286 0.0527 2.5648
cs of the hydrogels network at (a) 4, (b) 22 and (c) 37 °C was determined. All the hy-ydrogels show temperature dependent swelling behavior that is suppressed by the ad-chain movements. (c) The transport properties of solvent within the nanocompositeonstant (k) and the characteristic exponent (n) that describe the mode of solvent trans-lues of “n” and “k”.
PE
G H
ydro
gel
s1%
Sili
ca5%
Sili
ca10
%S
ilica
10 µm 2 µm
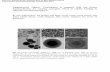
Fig. 3. Effect of silica nanospheres on morphology of PEG hydrogels. Cryo-SEM images of PEG and PEG-silica nanocomposite samples show porous microstructures. Addition of silicadoes not result in significant changes of pore size or shape. At higher silica concentrations (10%), aggregates were observed.
1803A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
(Fig. 1a). The addition of silica nanosphere to the prepolymer solutionsresults in a decrease in optical transmittance. This is due to the formationof microsized silica aggregates that scatter visible light. The prepolymersolutions can be easily injected through 22-gauge needles indicating ap-plicability for minimally invasive therapies. Upon exposure to UV, theprepolymer solutions readily form covalently crosslinked networks.While, the PEG hydrogels were transparent, the addition of silica ledto the formation of translucent hydrogels (Fig. 1b). PEG-based hydrogelscontaining different amounts of silica nanosphere (0, 1, 2.5, 5 and 10%)were prepared (Fig. 1c) and then their physical, chemical and some cel-lular adhesion characteristics were evaluated.
3.1. Effect of silica nanospheres on hydration kinetics of PEG hydrogels
Hydration properties of the hydrogel networks play a critical role indetermining the applicability in biotechnological and biomedical appli-cations. Hydrogels with a high swelling ratio can be used as an activematrix that absorbs fluids. The diffusion of entrapped macromoleculesalso depends on the hydration properties of the polymeric network.Since PEG is a highly hydrophilic polymer, it is expected that PEG-basednanocompositeswill readily hydratewhen exposed to an aqueous envi-ronment. The hydration properties of PEG-silica hydrogels were evalu-ated by submerging the freeze-dried nanocomposites in PBS at different
0 1 2.5 5 100
5
10
15
20
25
30
35
Fra
ctur
e S
tres
s (k
Pa)
Silica (%)0 1 2.5 5 10
200
250
300
350
400
Fra
ctur
e S
trai
n (%
)
Silica (%)
0 1 2.5 5 100
10
20
30
40
50
Fra
ctur
e E
nerg
y (k
Pa)
Silica (%)0 1 2.5 5 10
0
4
8
12
16
20
*
*
Mod
ulus
(kP
a)
Silica (%)
ToeLinear
*
0 100 200 300 4000
5
10
15
20
25
30
35
40 Silica
0% 1% 2.5% 5% 10%
Str
ess
(kP
a)
Strain (%)
Silica
a b
c
d
Fig. 4. Effect of silica nanospheres on mechanical properties of PEG hydrogels. Addition of silica significantly influences mechanical properties. (c) The Young modulus of thehydrogels is calculated from tow (0–50% strain) and linear (200–250% strain) regions. Increase in silica concentration results in an increase in modulus and fracture energy indi-cating reinforcement of the hydrogel network. (d) No significant increase in fracture strain was observed within samples. While, fracture stress increases with an increase in silicaconcentration.
1804 A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
conditions: (a) storage (4 °C), (b) handling (22 °C), and (c) physiolog-ical conditions (37 °C). All hydrogel compositions reached an equilibri-um hydration degree within 12 h (Fig. 2a). The hydration degreedecreasedwith an increase in silica concentration. For samples containing10% silica, more than a 5-fold decrease in saturated hydration degreewasobserved at 4 and 22 °C, andmore than a 3-fold decrease in hydrationdegree was observed at 37 °C (Fig. 2b) when compared to pure PEGhydrogels. The saturated hydration degree of hydrogels stronglydepended on the cross-linking density of the polymeric matrix andnanosphere–polymer interactions. As the amount of polymer was keptconstant in all of the hydrogels, it is evident that the addition of silica re-sults in significantly higher polymer–nanoparticle interactions that de-creased the saturation hydration degree. This was further verified byre-evaluating the swelling kinetics of polymeric networks (excludingnanoparticle weight) from nanocomposite hydrogels. A similar trendwas observed at all the temperatures which further reinforced the factthat the addition of silica restricts chainmovement and saturation hydra-tion degree of polymeric network.
The swelling ratio is an indirect representation in terms of total watercontent. For example, nanocomposite networkswith a swelling ratio of 5contain more than 80% of solvent, whereas the nanocomposites with aswelling ratio of 20 contain more than 95% solvent. From these results,
we conclude that silica significantly influences thewater uptake capacityof the PEG network. The rate of water uptake by the hydrogel networkcan be used to determine diffusion kinetics. The transport propertiesand diffusion coefficients of hydrogel networks at different temperatureswere evaluated by fitting the initial swelling data to Fick's equation. Forall samples, the diffusion coefficients (n) was less than 0.5 at all temper-atures. This suggests that the hydrogels show Fickian or Case I transportbehavior possibly due to high polymer chain mobility (Fig. 2c). Theaddition of silica led to an increase in diffusion co-efficient of thePEG hydrogel matrix, which is mainly attributed to the higherpolymer–nanoparticle interactions. In addition, a strong influenceof temperature on diffusion co-efficients of these networks was ob-served. At higher temperatures (37 °C), the hydrogel network promotehigher diffusion compared to low temperatures (4 °C). Similar re-sults were reported by Pandis who showed that the addition of silicananoparticles to polymeric hydrogels decreased the water uptakeability of the nanocomposite hydrogels [59].
3.2. Effect of silica on morphology of PEG hydrogels
The physical and chemical properties of nanocomposite hydrogelsdepend on the dispersion of nanoparticles within the polymer network.
0 1 2.5 5 1075
80
85
90
95
100
Rec
over
y (%
)
Silica (%)
ba
d
0 10 20 30 40 500
4
8
12
16
20
Compressive Strain (%)
0 10 20 30 40 500
4
8
12
16
20
Com
pres
sive
Str
ess
(kP
a)
Compressive Strain (%)
0 10 20 30 40 500
4
8
12
16
20
Compressive Strain (%)
0 10 20 30 40 500
4
8
12
16
20
Compressive Strain (%)
PEG
PEG-1%Silica
PEG-10%Silica
PEG-5%Silica
c
Silica (%)CompressiveModulus (kPa)
PEG 7.9 ±4.3
1% Silica 8.6 ± 3.7
2.5% Silica 8.5 ± 1.1
5% Silica 11.7 ± 1.4
10%Silica 11.6 ± 3.3
Com
pres
sive
Str
ess
(kP
a)C
ompr
essi
ve S
tres
s (k
Pa)
Com
pres
sive
Str
ess
(kP
a)
Fig. 5. Effects of silica on compressive properties of PEG hydrogels. (a) Qualitative images showing the highly elastic nature of the nanocomposite hydrogels. (b) Compressive prop-erties of the hydrogels with different compositions. (c) Table summarizing compressive moduli. (d) The percentage recovery was calculated by the ratio of area under the unloadingand loading curves. The PEG hydrogel control show an elastic behavior with more than 95% recovery. The addition of silica to the PEG hydrogel decreases the recovery suggestingviscoelastic properties.
1805A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
For example, fully dispersed nanocomposites have very different prop-erties compared to pure polymer or microcomposites. Moreover, inter-actions between polymer chains and silica nanospheres can influencemicrostructural characteristic and mechanical properties. The micro-structural evaluation of the hydrogel network is important becausepore size plays a significant role in the diffusion of nutrients and othermacromolecules through the hydrogel network.
We performed a microstructural evaluation of hydrogel networks byusing cryogenic temperature-scanning electron microscopy (cryo-SEM).This technique allows us to image freeze-fractured hydrogels after etch-ing. The results help us to understand how silica nanospheres are dis-persed within the PEG hydrogels and how silica influences the nativehydrogel architecture. Overall, the results show that pure PEG hydrogelshave highly porous and interconnected networks with pore sizes in therange of ~1–2 μm (Fig. 3). On the length scale investigated here,the addition of silica nanospheres does not seem to have any signif-icant effect on the pore size of the PEG network. However, we ob-served an increase in the aggregation of silica nanospheres with anincrease in silica concentration. At higher magnification, we observedthat pore walls also contain silica nanospheres. This indicates that silicanot only build aggregates but a part of silica is well dispersed and inter-acts with the polymer.
3.3. Effect of silica on tensile properties of PEG hydrogels
An evaluation of mechanical properties of the hydrogel networksis important to biomaterials engineering. The effect of silica on thehydrogel mechanical properties was evaluated by using the uniaxialtensile tests and unconfined compression tests. All hydrogels showedsigmoidal stress–strain curves with both low modulus and high re-versible deformation that are typically observed for elastomeric ma-terials (Fig. 4a). The sigmoidal curve can be divided into the toeregion (0–50% strain), knee region (50–150% strain), and a linear region(150–300% strain) (Fig. 4b). The initial toe region can be correlated torubbery elasticity or the energy required to stretch entangled polymerchains, while the linear region can be correlated to high extension andstress build-up during stretching of chains. The modulus from boththe toe and linear regionswas calculated to evaluate the effect of the ad-dition of silica onmechanical properties (Fig. 4c). There is no significantdifference in the modulus calculated from the toe and the linear regionfor the pure PEG hydrogel control. The addition of silica (>2.5% silicananospheres) results in significant differences between moduli from dif-ferent regions.Moreover, the addition of silica to hydrogels leads to signif-icantly higher tensile moduli when compared with the moduli of purePEG hydrogels (*pb0.05). These results suggest that silica reinforces the
Day 1 Day 3 Day 70
1
2
3
4
5
Inte
nsity
(a.
u)
PEG1% Silica5% Silica10% SilicaTCPS (Control)
c d
b PEG Hydrogels 1%Silica 5%Silica 10%Silica TCPS
Metabolic Activity
0 1 5 10 TCPS0
20
40
60
80
100
*
*
Initi
al C
ell A
dhes
ion
(a.u
)
Silica (%)
*
a
PEG PEG-5% Silica PEG-10% Silica
Silica
Fig. 6.Effect of silica on cell adhesion, spreading andproliferation of hydrogel surface. (a) A schematic showing addition of silica nanospheres improves cell adhesionproperties. (b) Fluorescenceimages showing the effect of silica addition to PEG hydrogels on cell adhesion and spreading. The PEGhydrogel control showsminimal cell adhesion (scale bar=200 μm). The addition of smallamounts of silica significantly enhances cell adhesion. Higher silica concentrations lead to cell adhesionwithwell organized cytoskeletons. TCPS stands for tissue culture polystyrene. (c) Initialcell adhesionwas quantified by using ImageJ (NIH). (d) Effect of silica on long term cellular functionwas determined bymonitoringmetabolic activity of cells by using anMTS assay. Addition ofsilica results in an increase in metabolic activity of attached cells.
1806 A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
polymer network and restricts chainmovements during tensile deforma-tion. The stress–strain curves also indicate that the addition of silica signif-icantly improves toughness (fracture energy), and fracture stress (Fig. 4cand d). However, no significant improvement was observed in the ulti-mate strain (fracture strain). Overall, these results lead us to the conclu-sion that silica nanospheres act as a physical reinforcement. Thepresence of the silica nanospheres improve the mechanical strengthof the PEG hydrogel network without compromising extensibility.
Similar results were reported in literatures on different hydrogelsystems. For example, Yang showed that the addition of silicananoparticles to poly(acrylic acid) hydrogels resulted in an increase inthe modulus and is directly dependent on the amount of silica andwater contents [48]. In a similar approach, Wang reported supertough hydrogels from poly(2-acrylamido-2-methylpropane sulfonicacid)/polyacrylamide (PAAm) reinforcedwith silica nanoparticles [53].Moreover, other recent studies indicated that PEG hydrogels can bephysically reinforced with other types of nanoparticles (such as hy-droxyapatite [32] and layered silicates [54]).
3.4. Effect of silica on compressive properties of PEG hydrogels
In considering hydrogels for soft tissue engineering, it is important toaddress the stability of the polymeric network under stress-relaxation cy-cles. We observed that all the nanocomposite hydrogels can be subjectedto repetitive cyclic loading (Fig. 5a and b). Although the addition of silica
results in an increase in the compressive modulus of the hydrogelnetwork, no significant difference between the groups was observed(Fig. 5c). PEG hydrogel control shows highly elastomeric behaviorwhen subjected to repeated cyclic stress (up to 50% strain) and therecoverywasmore than 90% for all the compressive cycles. The additionof silica significantly reduced the percentage recovery (Fig. 5d).With the addition of 10% of silica, for example, the percentage recov-ery drops to 76%. This can be attributed to induced viscoelasticityand possibly to restricted chain movement in the presence of silica.Thus the silica reduces the elastic properties of PEG by disruptingthe covalently crosslinked PEG network. Similar results were demon-strated with PEG-hydroxyapatite and PEG–silicate-based hydrogels[32,54]. Overall, the combination of silica nanospheres and PEGresulted in the development of hydrogels with tunable mechanicalproperties. Hydrogels with biologically relevant mechanics can beobtained by means of physiologically compatible procedures,addressing a broad range of applications within the tissue-engineeringframework.
3.5. Effect of silica on cell adhesion, spreading, and proliferation
Hydrogels have attracted great interest in biotechnological andbiomaterial developments because of their high water content, bio-compatibility and mechanical properties resembling those of naturaltissues [55,56]. Designing hydrogel platform with appropriate clues
1807A.K. Gaharwar et al. / Materials Science and Engineering C 33 (2013) 1800–1807
for regulating cellular behavior through biochemical and biomechanicalpathways is increasingly appreciated for biotechnological and biomate-rials engineering. For example, cellular behavior can be controlled orguided by simply changing some of the properties of hydrogels. One ap-proach to control cell adhesion is to incorporate adhesive peptides suchas Arg-Gly-Asp (RGD) that promote cell adhesion [13]. In another ap-proach to control cellular behavior, Schmidt showed that cell adhesioncan be tuned by incorporating gold nanoparticles into soft polymericfilms [57]. The gold nanoparticles improve mechanical properties andincrease the surface roughness which ultimately aids in cellular adhe-sion. In a similar approach, Gaharwar used to control cell adhesionand spreading in the PEG network [58].
To evaluate the effect of silica on cell adhesion, spreading and prolif-eration, NIH3T3 fibroblast cells were seeded on the surfaces of hydrogels(Fig. 6). The PEG hydrogel control does not promote cell adhesion asexpected, but the addition of silica induces cell adhesion. Cells attachedto PEG surfaces assume a round shape morphology due to poorcell–matrix interactions. The addition of silica provided cell adhesionsites on the hydrogel surfaces via the attachment of media proteins tothe silica surfaces. The effect of silica on cellular activity was moni-tored by investigating the metabolic activity of the attached cells(Fig. 6d). The metabolic activity of cells was determined and theresults indicated that the proliferation of cells strongly depends onsilica concentration. Adding silica results in a significant increase inmetabolic activity of cells from day 1 to day 7 on hydrogels containing10% silica. Thus, cell–hydrogel interactions can be tuned by providingadhesion sites through silica and by reinforcing and strengthening thehydrogel network.
4. Conclusion
The addition of silica nanospheres to PEG hydrogels improved me-chanical strength and decreased hydration kinetics. More than a 4-foldincrease in tensile modulus and a 3-fold increase in fracture strengthwere observed by adding 10% of silica. The compression of hydrogelssuggests elastic and viscoelastic behaviors and the ability to recoverback after deformation. Cell adhesion on hydrogel surface shows that sil-ica provides sites for cell adhesion, and allows spreading, and prolifera-tion. These properties can be tuned to obtain hydrogels with controlledcell adhesion to be used for various biomedical and biotechnologicalapplications.
References
[1] N.A. Peppas, J.Z. Hilt, A. Khademhosseini, R. Langer, Adv. Mater. 18 (2006)1345–1360.
[2] D. Seliktar, Science 336 (2012) 1124–1128.[3] B.V. Slaughter, S.S. Khurshid, O.Z. Fisher, A. Khademhosseini, N.A. Peppas, Adv.
Mater. 21 (2009) 3307–3329.[4] C.M. Elvin, T. Vuocolo, A.G. Brownlee, L. Sando, M.G. Huson, N.E. Liyou, et al., Bio-
materials 31 (2010) 8323–8331.[5] J.L. West, J.A. Hubbell, Biomaterials 16 (1995) 1153–1156.[6] Y. Yeo, D.S. Kohane, Eur. J. Pharm. Biopharm. 68 (2008) 57–66.[7] Y. Luo, K.R. Kirker, G.D. Prestwich, J. Control. Release 69 (2000) 169–184.[8] S. Sant, S.L. Tao, O.Z. Fisher, Q. Xu, N.A. Peppas, A. Khademhosseini, Adv. Drug
Deliv. Rev. 64 (2012) 496–507.[9] R. Censi, P. Di Martino, T. Vermonden, W.E. Hennink, J. Control. Release 161
(2012) 680–692.[10] K.T. Nguyen, J.L. West, Biomaterials 23 (2002) 4307–4314.
[11] L.S. Nair, C.T. Laurencin, M. Tandon, Injectable Hydrogels as Biomaterials, JohnWiley & Sons, Inc., 2010
[12] L. Yu, J. Ding, Chem. Soc. Rev. 37 (2008) 1473–1481.[13] J.A. Burdick, K.S. Anseth, Biomaterials 23 (2002) 4315–4323.[14] R.H. Schmedlen, K.S. Masters, J.L. West, Biomaterials 23 (2002) 4325–4332.[15] J.W. Nichol, S.T. Koshy, H. Bae, C.M. Hwang, S. Yamanlar, A. Khademhosseini, Bio-
materials 31 (2010) 5536–5544.[16] J. Baier Leach, K.A. Bivens, C.W. Patrick Jr., C.E. Schmidt, Biotechnol. Bioeng. 82
(2003) 578–589.[17] J.A. Burdick, C. Chung, X. Jia, M.A. Randolph, R. Langer, Biomacromolecules 6
(2005) 386–391.[18] O. Jeon, K.H. Bouhadir, J.M. Mansour, E. Alsberg, Biomaterials 30 (2009) 2724–2734.[19] B.G. Amsden, A. Sukarto, D.K. Knight, S.N. Shapka, Biomacromolecules 8 (2007)
3758–3766.[20] N.A. Peppas, R. Langer, Science 263 (1994) 1715–1720.[21] B.D. Ratner, S.J. Bryant, Annu. Rev. Biomed. Eng. 6 (2004) 41–75.[22] R.A. Hule, D.J. Pochan, MRS Bull. 32 (2007) 354–359.[23] A. Okada, A. Usuki, Macromol. Mater. Eng. 291 (2006) 1449–1476.[24] D.R. Paul, L.M. Robeson, Polymer 49 (2008) 3187–3204.[25] R.A. Vaia, H.D. Wagner, Mater. Today 7 (2004) 32–37.[26] C.-J. Wu, A.K. Gaharwar, P.J. Schexnailder, G. Schmidt, Materials 3 (2010)
2986–3005.[27] D. Das, T. Kar, P.K. Das, Soft Matter 8 (2012) 2348–2365.[28] Karen I. Winey, Richard A. Vaia, MRS Bull. 32 (2007) 314–322.[29] L.L. Hench, J. Am. Ceram. Soc. 74 (1991) 1487–1510.[30] L.L. Hench, J.M. Polak, Science 295 (2002) 1014–1017.[31] P. Schexnailder, G. Schmidt, Colloid Polym. Sci. 287 (2009) 1–11.[32] A.K. Gaharwar, S.A. Dammu, J.M. Canter, C.-J. Wu, G. Schmidt, Biomacromolecules
12 (2011) 1641–1650.[33] A.K. Gaharwar, V. Kishore, C. Rivera, W. Bullock, C.-J. Wu, O. Akkus, et al.,
Macromol. Biosci. 12 (2012) 779–793.[34] A.K. Gaharwar, P. Schexnailder, V. Kaul, O. Akkus, D. Zakharov, S. Seifert, et al.,
Adv. Funct. Mater. 20 (2010) 429–436.[35] Q. Jin, P. Schexnailder, A.K. Gaharwar, G. Schmidt, Macromol. Biosci. 9 (2009)
1028–1035.[36] A.K. Gaharwar, P.J. Schexnailder, A. Dundigalla, J.D.White, C.R. Matos-Pérez, J.L. Cloud,
et al., Macromol. Rapid Commun. 32 (2011) 50–57.[37] A.K. Gaharwar, P.J. Schexnailder, Q. Jin, C.-J. Wu, G. Schmidt, ACS Appl. Mater. In-
terfaces 2 (2010) 3119–3127.[38] P.J. Schexnailder, A.K. Gaharwar, R.L. Bartlett Ii, B.L. Seal, G. Schmidt, Macromol.
Biosci. 10 (2010) 1416–1423.[39] L. Wang, W. Zhao, W. Tan, Nano Res. 1 (2008) 99–115.[40] D. Knopp, D. Tang, R. Niessner, Anal. Chim. Acta 647 (2009) 14–30.[41] S. Selvan, Biointerphases 5 (2010) FA110–FA115.[42] V. Mamaeva, C. Sahlgren, M. Lindén, Adv. Drug Deliv. Rev. (2012), http://dx.doi.org/
10.1016/j.addr.2012.07.018.[43] P. Yang, S. Gai, J. Lin, Chem. Soc. Rev. 41 (2012) 3679–3698.[44] J.L. Vivero-Escoto, I.I. Slowing, B.G. Trewyn, V.S.Y. Lin, Small 6 (2010) 1952–1967.[45] A. Bitar, N.M. Ahmad, H. Fessi, A. Elaissari, DrugDiscov. Today 17 (2012) 1147–1154.[46] N.J. Halas, ACS Nano 2 (2008) 179–183.[47] J. Cai, S. Liu, J. Feng, S. Kimura, M. Wada, S. Kuga, et al., Angew. Chem. Int. Ed. 51
(2012) 2076–2079.[48] J. Yang, X.-P. Wang, X.-M. Xie, Soft Matter 8 (2012) 1058–1063.[49] M. Takafuji, S.-Y. Yamada, H. Ihara, Chem. Commun. 47 (2011) 1024–1026.[50] A. D'Agostino, M. Errico, M. Malinconico, M. De Rosa, M. Avella, C. Schiraldi, J. Mater.
Sci. Mater. Med. 22 (2011) 481–490.[51] G.R. Beck Jr., S.-W. Ha, C.E. Camalier, M. Yamaguchi, Y. Li, J.-K. Lee, et al., Nanomed.
Nanotechnol. Biol. Med. 8 (2012) 793–803.[52] C.-J. Wu, A.K. Gaharwar, B.K. Chan, G. Schmidt, Macromolecules 44 (2011)
8215–8224.[53] Q. Wang, R. Hou, Y. Cheng, J. Fu, Soft Matter 8 (2012) 6048–6056.[54] A.K. Gaharwar, C.P. Rivera, C.-J.Wu, G. Schmidt, Acta Biomater. 7 (2011) 4139–4148.[55] K.Y. Lee, D.J. Mooney, Chem. Rev. 101 (2001) 1869–1879.[56] J.A. Rowley, G. Madlambayan, D.J. Mooney, Biomaterials 20 (1999) 45–53.[57] S. Schmidt, N. Madaboosi, K. Uhlig, D. Köhler, A. Skirtach, C. Duschl, et al., Langmuir
28 (2012) 7249–7257.[58] A.K. Gaharwar, P.J. Schexnailder, B. Kline, G. Schmidt, Acta Biomater. 7 (2011)
568–577.[59] C. Pandis, A. Spanoudaki, A. Kyritsis, P. Pissis, J.C.R. Hernández, J.L. Gómez Ribelles,
M. Monleón Pradas, J. Polym, Sci. Part B: Polym. Phys. 49 (2011) 657.
Related Documents