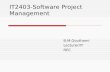
Chemistry Half Yearly Exam Study THE CHEMICAL EARTH 3. Identify the difference between elements, compounds, and mixtures in terms of particle theory. See Definition of element compound and mixture Matter (has mass and occupies space) Homogeneous Heterogeneous (copper, sugar, whisky, can be separated into (muddy water, concrete, strawberry jam, salt solution, air) uniform marmalade and wood) variable composition and properties composition and properties throughout throughout always Pure Substances Mixtures (Salt solution, (copper, sugar, water, oxygen can be separated whisky, air) variable nitrogen, alcohol) constant physically into composition composition

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Chemistry Half Yearly Exam Study
THE CHEMICAL EARTH
3. Identify the difference between elements, compounds, and mixtures in terms of particle theory.
See Definition of element compound and mixture Matter
(has mass and occupies space)
Homogeneous Heterogeneous(copper, sugar, whisky, can be separated into (muddy water, concrete, strawberry jam,salt solution, air) uniform marmalade and wood) variable composition and properties composition and propertiesthroughout throughout
always
Pure Substances Mixtures (Salt solution,(copper, sugar, water, oxygen can be separated whisky, air) variable nitrogen, alcohol) constant physically into compositioncomposition
Elements Compounds(Copper, helium, neon, nitrogen, oxygen) (sugar, water, salt, baking soda and alcohol) (2 ornot separable into simpler substances more elements chemically combined in fixed
proportions
4. Identify that the biosphere, lithosphere, hydrosphere and atmosphere contain examples of mixtures of elements and compounds.
AtmosphereElements % by mass as compounds/elements CompoundsNitrogen 75.3% waterOxygen 23.2% carbon dioxide

Argon 1.3% nitrogen oxides NOx
Carbon 0.1% MethaneNeon 0.0015% sulphur oxides SOx
Helium 0.0005% carbon monoxideElements existing alone: Argon, Oxygen, Neon, Nitrogen
LithosphereElements % by mass as compounds/elements CompoundsOxygen 47% oxides- silicon, iron, aluminiumSilicon 28% sulfides- lead, silver, zincAluminium 8% carbonates- calcium, magnesiumIron 5% silicates- pyroxenesCalcium 4% silicon dioxideSodium 3% aluminosilicatesPotassium 3% SulfatesMagnesium 2% ChloridesOthers <1%Element existing alone: Silver, Platinum, Gold, Copper, Tin, Carbon
HydrosphereElements % by mass as compounds/elements CompoundsOxygen 85.7% Carbon dioxideHydrogen 10.8% chlorides- sodium, magnesium, calciumChlorine 1.9% sulfates- sodium, magnesium, calciumSodium 1.1%Others <1%Elements existing alone: Oxygen, Nitrogen
BiosphereElements % by mass as compounds/elements CompoundsOxygen 60% sugarCarbon 21% carbohydrates (Cx Hx Ox) Hydrogen 11% starchNitrogen 3.5% celluloseCalcium 2.5% glycogenPotassium 1.2%
5. Identify and describe procedures that can be used to separate naturally occurring mixtures of:
1. solids of different sizes2. solids and liquids3. dissolved solids in liquids4. liquids5. gases

1 Mixtures in which the particles of the different substances have different sizes can be separated by sieving. In the laboratory and in industry sieves are used to separate small particles from large ones. At quarries fine sand, needed for making concrete and mortar, is separated from the coarser material by sieving. This process is also used to sort foodstuffs and in water treatment.
2 Mixtures of solids and liquids are commonly separated by filtration. The liquid or solution passes through the paper while the suspended solid remains on the top of the filter paper. The liquid or solution which passes through the filter paper is called a filtrate. Sand can be separated from sea water in this way. It is also used in the purification of our water supply and to remove solids from petrol in car engines.Sometimes if the solid is present as coarse or very dense particles, sedimentation and decantation can be used. Sedimentation is the process in which solids settle to the bottom of a container. Decantation is the process of carefully pouring off the liquid and leaving the solid undisturbed at the bottom of the container. Pouring tea off tea leaves is decantation. Sedimentation and decantation is used in the purification of water supplies and in waste water treatment. It is also used to separate cream from milk.
Solids- liquids3 When a solid is dissolved in a liquid, the solid and liquid can be separated by eporating (vaporising) the liquid. We can do this either by boiling the solution or by just evaporating it so that some of the particles ‘escape’ from the surface of the liquid. Chemists frequently use the expression evaporate to dryness, which means heating a solution in an evaporating basin to drive off all the solvent. Evaporating to dryness is a common way of obtaining a solid from a solution. Salt is obtained commercially from sea water in this way. It is also used in mineral extraction and gold amalgamation.
Solids- liquids and liquids- liquids4 Distillation is a method of separating liquids from solutions or of purifying one liquid. It involves boiling the material and condensing the resulting vapour back to a liquid in a different part of the apparatus.The mixture or impure liquid, for example seawater, is placed in the flask and heated to boiling. The liquid changes to vapour, rises up the neck of the flask and diffuses down the side arm and into the water- cooled condenser, where the vapour is cooled and condensed back to a liquid which is collected in the beaker. The liquid collected from a distillation is called a distillate.If the impurities are non- volatile (as in the case with sea water), pure liquid is collected (pure water from sea water). Distillation is also used in the manufacture of spirits such as brandy, whisky and rum. These spirits are obtained by the distillation of wines, fermented grains and sugar cane respectively. It is also used in water treatment and to obtain salt from salt water while retaining the water.
Liquids- liquids (close boiling points)

Separating liquids by distillation when their boiling points are fairly close together can be done in specially designed equipment: the process is called fractional distillation. This arrangement allows for repeated condensations and vaporisations up the column effectively giving many separate distillations (typically 10 to 100). This means that eventually a pure sample of the more volatile substance in the original mixture emerges from the top of the column. In the petroleum industry, repeated fractional distillation is used to separate the components of crude oil into aviation spirit, petroleum, kerosene, lubricants, waxes and asphalt. Fractional distillation is also used to separate mixtures of gases for example, oxygen and argon from air. The Oxygen is used for medical purposes and the Argon is used for filling light bulbs.
Separating immiscible liquidsImmiscible liquids are generally separated by using a separating funnel. This pear- shaped piece of apparatus tapers to a narrow tube just above the stopcock. This shape allows us to run out the bottom liquid without getting it contaminated with any of the top one. A mixture of petrol and water can be separated in this way.
Separation based on solubilityMixtures of solids can be easily separated if one solid is soluble in a particular solvent while the others are not. Sufficient solvent is added to the mixture to dissolve the soluble component then the insoluble components are filtered off. The soluble solid is recovered by evaporating the filtrate to dryness. A mixture of salt and sand can be separated this way by adding sufficient water to dissolve the salt then filtering off the sand.
5 gas mixtures are generally separated by using either differences in boiling points or differences in solubilities in liquids such as water. Oxygen for medical uses and argon for filling light bulbs are obtained from air by fractional distillation. Fractional distillation rather than simple distillation is used because the boiling points of nitrogen, oxygen and argon are so close together. First the air is liquefied (by cooling it to below -196 degrees Celsius), then the mixture is fractionally distilled.
CrystallisationCrystallisation depends on the components of the mixture having different solubilities in a selected liquid, usually water. Crystallisation is used in the production of sugar from sugar cane and sugar beet. Mixtures containing 2 or more soluble components can also often be separated by solution, filtration and fractional crystallisation, provided the components have different solubilities at different temperatures. For example, a mixture of salt and baking soda can be separated by dissolving it is hot water and then cooling the resultant solution. While both substances are soluble in hot water, the baking soda is much less soluble in cold water and most of it will crystallise from the solution when it is cooled. This can then be filtered, leaving all the salt and only a small amount of baking soda in solution. Crystallisation is used in the production of silicon crystal wafers, powder salt for food, and macroscopic crystal production.
CentrifugationCentrifugation separates mixtures of chemicals using a spinning motion in a machine

called a centrifuge. It is commonly used to separate liquids from solids in a manner similar to, but faster than, sedimentation. A common example is the spin dryer in a washing machine. The method may also be used to separate finely divided solid particles present in a liquid mixture. Centrifuges are used widely in industry, for example, to separate cream from milk and plasma from blood. It is also used in separating textiles.
ChromatographyChromatography is a relatively recent development but is particularly useful for separating components that are represented in small quantities of mixtures. There are several different chromatographic techniques, including column chromatography, thin layer chromatography, and gas chromatography. In each of these techniques the mixture is passed over the surface of an inert substance such as alumina, silica or special paper. The separation of the components in the mixture occurs because the components absorb, or cling, to the surface of the inert substance with different strengths.
Column chromatography can be used, for example, to separate the various pigments found in plant matter. A solution of the pigments is passed through a column packed with alumina. The different pigments will pass through the column at different speeds, depending on the strength with which they adhere to the alumina. The less strongly absorbed components pass more quickly through the column than those that are absorbed more strongly. The pigments can be collected as they wash out of the column.
In paper chromatography a solution of the mixture is allowed to move up strips of special filter paper. Again the components in the mixture that adhere more strongly to the paper will move more slowly up the paper strip. This technique is sometimes used to detect food additives and dyes or particular drugs that are only present in small quantities in mixtures.
Thin layer chromatography is very similar to paper chromatography. The mixture moves up a plate made from glass or plastic and coated with a thin layer of a fine powder such as aluminium oxide. It is a more expensive process than paper chromatography but is faster and capable of detecting smaller quantities of substances.
Gas chromatography is used to detect the components present in a gas or vaporised mixture. The gas mixture is passed through a tube containing a particular solid such as carbon or silica. The different components present pass through the tube at different rates and can be detected as they emerge form the tube. The components of natural gas can be analysed in this way.
Magnetic separationSome components of a mixture can be separated from the mixture because they are magnetic. Iron- containing minerals such as magnetite are separated from iron ore in this way. The crushed ore sample is passed between the poles of a strong electromagnet, thus separating out the magnetic material and allowing the crushed rock particles to be removed. Magnetic separation is also used to separate some of the composition of mineral sands.

Assess separation techniques for their suitability in separating examples of earth materials, identifying the differences in properties which enable these separations
Separation method property used in the separation
Sieving particle size--- separate fine sand from coarser material at quarries for making concrete and mortar.
Vaporisation (evaporating or boiling) liquid has a much lower boiling point than the solid dissolved in it--- separating salt from salt- water.
Distillation moderate difference in boiling points--- purifying dirty water and manufacturing whisky, rum and brandy
Fractional distillation significant but small difference in boiling points--- separate fractions of petroleum, liquids of different boiling point
Filtration one substance a solid (undissolved), the other a liquid or solution--- used for brewing coffee and treatment of sewerage.
Adding a solvent then filtration one substance is soluble in the chosen solvent, while the others are insoluble (insoluble- filtration, soluble- evaporisation) e.g. separating a mixture of sand and salt.
Crystallisation depends on solubility at different temperatures--- it produces a product that is free from impurity, salt sugar cane, juice.
Chromatography depends on the strength that a substance will adhere to an inert substance--- used in forensic science and biology to identify compounds and separate complex mixtures.
Centrifugation particle size, density--- separate components of blood, separate water from washing.
Froth Flotation polarity, gangue is polar--- mineral refining separating lead sulphide from rock

Settling and decantation particle size density--- tea from tea leaves.
Magnetic separation Magnetism--- recycled waste treatment of sewerage.
Using a separating funnel components are immiscible liquids e.g. separating a mixture of petrol and water
6. Describe situations in which gravitational analysis supplies useful data for chemists and other scientists.
7. To decide whether a newly discovered mineral deposit contains a sufficiently high percentage of the required compound to make its extraction from that deposit economically viable.
8. To determine the composition of soil in a particular location to see if it is suitable for growing a certain crop.
9. To determine the amounts of particular substances present in water or air to decide how polluted the samples are.
10. To decide whether a particular commercial mixture being sold has the same percentage composition as a similar mixture being marketed by a rival company.
11. The measurement of the essential elements in plant foods (e.g. NPK ratio Nitrogen- phosphorous- potassium.)
12. For the composition of food products and additives e.g. salt, sugar, fat, protein.
13. Explain the relationship between the reactivity of an element and the likelihood of its existing as an uncombined element.
Apart from a few elements, most elements occur as compounds. This is because most elements are chemically reactive: that is when they come into contact with certain other elements they react to form compounds. The general rule is that the more reactive an element is, the less chance there is of finding it in the Earth as an uncombined element.Sodium, potassium, calcium, magnesium, fluorine and chlorine which are all very reactive elements are never found as free elements. Elements in group 7 including fluorine, chlorine, bromine and iodine are non- metal elements that are highly reactive. That is why they are not found in nature as the free elements, instead they occur in combined form as compounds. Copper and sulphur which have moderate reactivity do exist naturally in some locations as uncombined elements, although they are more commonly found as compounds. Gold, platinum, argon and helium, which are extremely unreactive, occur naturally as uncombined elements. All the noble gases are extremely unreactive due to their filled outer electron shell and because of this are sometimes referred to as the inert gases.
14. Classify elements as metals, non- metals, and semi- metals according to their physical properties.

Metals
15. Relatively high densities (although lithium, sodium and potassium are less dense than water)
16. Good conductors of heat and electricity17. Malleable (can be beaten into sheets) and ductile (can be drawn into wires)18. Have a shiny surface when freshly cut or cleaned (lustrous)19. Relatively high melting points (although mercury and gallium have quite low
melting points).20. Solids at room temperature with the exception of mercury
Non- metals
21. State and form is variable, for example, oxygen is a gas, bromine a liquid and sulphur a solid.
22. Poor conductors of heat and electricity (except carbon in the form of graphite)23. Usually not lustrous24. Not malleable or ductile, often brittle25. Variable melting points
Semi- metals
26. Properties between those of metals and non- metals27. High melting point28. Usually high boiling point29. Not good conductors of heat and electricity30. They have a variable appearance
Property Metals Non- metals Semi- metals
Melting Point Usually high Usually low High
Boiling point Usually high Usually low Usually high
Electrical conductivity High Very low Low
Heat conductivity High Very low Low
Appearance Lustrous Usually not lustrous Variable
Examples Aluminium, lithium Chlorine, carbon Silicon, arsenic
31. Account for the uses of metals and non- metals in terms of their physical properties.
ELEMENT USE RELATED PHYSICAL PROPERTY

Iron (Fe) Building and bridge construction High tensile strength, malleable and tough, tensile structure
Carbon (C) Diamond used to cut glass, graphite used in lead pencils and as a lubricant
Hard Covalent Lattice structure of diamond, but graphite in pencils are soft and have a layered structure
Mercury (Hg) Used for making thermometers, barometers, diffusion pumps and mercury switches
Density, liquid state, heaviest known elemental liquid, expands when heated
Molybdenum (Mo)
Filaments material in electrical appliances
Malleable, ductile, high electrical conductivity
Antimony (Sb) Used in infrared detectors and diodes A semi- conductor
Boron (B) Boron filaments used in advanced aeroplane structure, fibre optic research
Lightweight, malleable
Helium (He) Used in filling balloons, blimps Less dense than air
Nitrogen (N) Used as liquid nitrogen fro the immersion, transportation and freezing of food, Cryopreservation of biological samples such as blood, as coolant for highly sensitive sensors
Ability to maintain temperatures far below the freezing point of water (it boils at 77K, which equals -196o C or -320o F)
Selenium (Se) Production of photocells and exposure meters for photographic use, as well as solar cells (gradually replaced by silicon based devices)
Photovoltaic action, where light is converted directly into electricity, and photoconductive action, where the electrical resistance decreases with increased illumination.
Aluminium (Al) Used in domestic utensils, drink can, saucepans, cooking foil, building construction as roofing and window frames, and in boat construction
Aluminium’s thermal conductivity, malleability and attractive lustre
Sulfur (S) Used in vulcanising rubber, and in the manufacture of sulfuric acid, fungicides, insecticides and hydrogen sulfite bleaches
Due to its abundance and reactivity
Titanium (Ti) Used in marine environment as propellers and other ship parts and in chemical factories where corrosive acids are used. Also, in lightweight, high strength alloys used in high- temperature environments, e.g. spacecraft and aircraft, pipes and linings for vats where acids are used
Titanium is resistant to corrosion, great strength, high melting point, low density, low reactivity, readily forms alloys

Zinc In galvanising iron, in the outer casing and negative electrode of dry cells, alloy (brass) used in fittings and fixtures
Fairly reactive but forms protective oxide layer, readily forms alloys.
Chromium (Cr) Plating other metals, as an additive in steel alloys, e.g. stainless steel
Shiny silver appearance, resists corrosion, readily forms alloys
32. Identify that matter is made of particles that are continuously moving and interacting.
Early in the 19th century, John Dalton proposed that a particular elements consists of identical atoms, and that different elements are made up of different types of atoms. At the time he formulated his theory, atoms were though of as hard, indivisible spheres which were the smallest particles capable of existence. Today it is accepted that the atom has a structure of its own, and is made up of 3 fundamental particles known as protons, electrons and neutrons. The masses and charges of these particles are summarised.
Particle Symbol Mass (Kg) Relative mass
Charge (C) Relative charge
ProtonNeutronElectron
Pne
1.673x10-27
1.675x10-27
9.110x10-31
11~1/1840
1.60x10-19
01.60x10-19
+10-1
Protons and neutrons have approximately the same mass, about 1.67x10-27 Kg. Electrons have a much smaller mass, about 1/1840th the mass of protons and neutrons. The mass of an electron is therefore almost negligible for most purposes when compared with the masses of protons and neutronsProtons and electrons have equal and opposite charges of 1.60x10-19 coulombs.It is conventional to describe protons as having a positive charge and electrons as being negatively charged. Because 1.60x10-19 coulombs (C) is a fundamental quantity of charge, the proton and electron are usually described as having charges of +1 and -1 respectively. Neutrons are uncharged (neutral).
The atom can be visualised in terms of 2 main regions. These regions are the nucleus and the surrounding space occupied by the electronsThe structure of the nucleus is as follows
33. It is the central part of the atom, and contains the protons and neutrons34. It has a positive charge equal to the number of protons35. It is exceedingly small compared with an atom. The diameter of an average
nucleus is 10-15m, compared with an average atomic diameter of 10-10 m. Thus, the atomic diameter is about 100000 times the diameter of the nucleus.
36. It contains over 99.9% of the mass of an atom. This is due to the relatively large masses of the proton and neutron compared with the mass of the electron.
37. It is exceptionally dense. This is due to its large mass and small volume.The arrangement of the electrons around the nucleus is as follows:
38. The electrons move through a relatively large space outside the nucleus.

39. They are kept moving around the nucleus by various forces.40. In an uncharged atom, the number of electrons equals the number of protons in
the nucleus.
The atomic number, Z, is the number of protons in the nucleus of an atom. This has a fixed value for atoms of any one element.
Matter is defined as anything that has mass and occupies space, such as wood, water, steel and air. Light, sound, and magnetic fields are examples of phenomenon that are not matter.
Matter commonly exists in 3 states- gas, liquid and solid. Water, for example, exists as steam, liquid water and ice.
41. Describe qualitatively the energy levels of electrons in atoms.
The fact that electrons (which are attracted to the nucleus) do not collapse on the nucleus indicates that the electrons possess energy- sufficient to resist the attraction towards the positive nucleus.Electrons in an atom exist in discrete energy levels which are called the first, second, third energy level and so on. Each electron in the first energy level has a certain constant amount of energy; each electron in the second energy level also has a fixed amount of energy but it is greater than that possessed by electrons in the first energy level. Similarly, the electrons in the third energy level have large amounts of energy still.The first energy level can accommodate only 2 electrons, the second can hold 8 and the third 18; in general the nth energy level can accommodate 2n2 electrons. Electrons must be in one energy level or another – they cannot have energies that are intermediate between 2 levels.Use periodic table or ‘step diagram’ to work out complex electron configurations.Chlorine (2.8.7.)
42. Describe atoms in terms of mass number and atomic number.
The atomic number, Z, of an element is the number of protons in the nucleus of an atom of that element. The mass number, A, is the number of protons plus neutrons in the nucleus of an atom of the species concerned. z
A X. phosphorous 3115P
43. Describe the formation of ions in terms of atoms gaining or losing electrons.
Ions are electrically charged species formed when atoms gain or lose electrons.CationsPositively charged ions, or cations, are formed when 1 or more electrons are removed from an atom. The resultant ion therefore has more protons than electrons. For example, when a sodium atom loses an electron the sodium ion formed has 11 protons and 10 electrons and has a relative charge of +1. The Sodium ion is represented as Na+.

These ions are formed in order for the atoms to achieve stable electron configuration.
AnionsNegatively charged ions, or anions, are formed when an atom gains 1 or more electron. An oxygen atom can gain 2 electrons to form an oxide ion, O2-. An oxide ion contains 8 protons and 10 electrons and has a relative charge of -2. These ions are formed in order for the atoms to achieve stable electron configuration.
Some elements can form more than 1 ion. Iron form forms 2 common ions namely iron(11), Fe2+ and iron(111), Fe3+. To distinguish between these ions, Roman numerals are placed in brackets after the element’s name to indicate the changes on the ions. The ions are called iron- two and iron- three respectively.
Polyatomic ionsPolyatomic ions are groups of atoms bonded to one another which have a net positive or negative charge. The carbonate ion, CO3
2-, is an example of a polyatomic ion. The carbon and oxygen atoms are joined by chemical bonds. The -2 indicates that there are 2 more electrons in the carbonate ion than the total number of protons possessed by the 4 atoms. These ions are formed in order for the atoms to achieve stable electron configuration.
44. Apply the periodic table to predict the ions formed by atoms of metals and non- metals.
The periodic table can be used to predict the charges on simple ions formed by many elements. The elements in group 1, with lithium at the top, all form ions with a charge of +1. The elements in group 2 form ions with a charge of +2, such as Mg2+ and Ca2+. Elements in group 3 usually form ions with a charge of +3. In group 4 the elements carbon and silicon tend to form ions; however, tin and lead do form +4 ions, although these elements also form +2 ions. The elements in group 5, with nitrogen at the top, usually form ions with a charge of -3. The elements in group 6 form -2 ions such as O2- and S2-, and those in group 7 -1 ions such as F-, Cl- and Br-. The elements in group 8 are inert and do not tend to form ions at all.The elements in the middle of the periodic table from Scandium across to zinc and going down the table, form positive ions, often with a charge of +2, although there are numerous exceptions. Many of these elements form ions with different charges, such as Fe2+ and Fe3+.However, while Groups 1 and 2 form only ionic compounds, Groups 6 and 7 also form covalent compounds.The transition metals all lose electrons to form positive ions (for example Ce3+, Fe2+, Cu2+, Ag+, Zn2+,), but it is not possible from a simple look at the periodic table to predict just how many electrons any particular atom will lose.Elements that are 3 places away from a noble gas may form ions: aluminium generally does (Al3+), boron never does, nitrogen and phosphorous do so on rare occasions.Another generalisation is that metals generally form positive ions while non- metals

(when they form ions) form negative ions.
45. Describe the formation of ionic compounds in terms of the attraction of ions of opposite charge.
Ionic compounds such as sodium chloride consist of oppositely charged ions held together by electrostatic attraction, to form a crystal lattice. The electrostatic attraction between the oppositely charged ions is called ionic bonding. Ionic bonds are strong, so ionic solids like sodium chloride and potassium fluoride are hard, brittle and difficult to cut.Ionic bonding is that type of chemical bonding which involves the outright transfer of electrons from one atom to another. In ionic compound, the basic particles that make up the compounds are ions. Because ionic compounds are electrically neutral, the number of positive charges must equal the number of negative charges.
1) Sodium and chlorine combine to form the compound, sodium chlorideThe sodium atom wants to lose 1 electron to obtain a complete outter shell and the chlorine atom wants to gain 1 electron to complete its outer shell. Hence 1 electron is transferred from a sodium atom to a chlorine atom. When the neutral chlorine atom gains an electron, it becomes a negative ion and is called the chloride ion. The sodium atom loses an electron and therefore forms a positive ion. There is strong electrostatic attraction between positive and negative ions. This is what holds the ions together in ionic bonding. Sodium chloride is therefore NaCl (to balance charges 1+ 1-). Crystals of sodium chloride consist of sodium atoms and chloride ions packed in an orderly fashion. The electrostatic attraction between pairs of oppositely charged ions extends throughout the whole crystal
2) Calcium and fluorine combine to form calcium fluorideEach calcium atom, loses 2 electrons, while each fluorine atom (2,7) gains 1 electron. Hence 2 fluorine atoms must combine with 1 calcium atom to form the ionic compound, CaF2. There is strong electrostatic attraction between positive and negative ions. This is what holds the ions together in ionic bonding.In ionic compounds there are no discrete molecules – just an infinite array of positive and negative ions.
46. Distinguish between molecules containing one atom (the noble gases) and molecules with more than one atom.
Many elements exist in nature as simple molecules. Molecules are particles that can move independently of each other. Molecules may contain 1 or more atoms. The noble gases, Helium, Neon, Argon, Krypton, Xenon and Radon, exist as independent atoms a molecule is defined as the smallest particle of a substance that is capable of separate existence, therefore these atoms are classified as molecules. The noble gases consist of monatomic (single atoms) molecules. Their formulae are the same as their chemical symbol. Most molecules consist of a group of 2 or more atoms held together by chemical bonds. Hydrogen gas (H2), for example, consists of molecules in which 2 hydrogen atoms are bonded together. The numerical subscript in the formula H2, ‘2’ in this case, indicates

the number of atoms of hydrogen present in the molecule. Similarly, the gases Oxygen (O2) and fluorine (F2), the liquid Bromine (Br2) and the solid iodine (I2) all exist as molecules in which 2 atoms are bonded together. Such molecules are described as diatomic molecules. Some elements exist as polyatomic molecules. One form of phosphorous, consists of molecules in which 4 atoms of phosphorous are bonded together (P4), while one form of sulphur consists of molecules which contain 8 sulfur atoms (S8).
47. Describe the formation of covalent molecules in terms of sharing of electrons.
2 chlorine atoms combine to form a chlorine molecule2 chlorine atoms can combine to form a chlorine molecule, Cl2, by sharing a pair of electrons with each atom contributing 1 electron to the shared pair. Each atom ‘considers’ that it ‘owns’ the shared pair and thus counts both members of the pair to determine that it has a noble gas configuration.This shared pair of electrons occupies a volume of space that surrounds both atoms. By moving around both nuclei these electrons hold the atoms together and so form a chemical bond. Each pair of shared electrons is called a covalent bond.
Hydrogen and oxygen combine to form waterOxygen tends to gain 2 electrons and hydrogen needs to gain 1 electron. Hence covalent bonds are formed between 1 oxygen atom and 2 hydrogen atoms to form the covalent molecule water. In this way each hydrogen atom and the oxygen atom ‘consider’ that they have achieved the noble gas configuration (because each atom ‘counts’ both electrons in the shared pair).
48. Identify the differences between physical and chemical change in terms of rearrangement of particles.
Physical changes are changes in physical properties such as volume or density, or changes in state such as changes from solid to liquid or from liquid to gas. Physical changes occur without a change in the composition of the particular substance.Chemical changes are those in which new substance with different compositions and properties are formed. The combustion of petrol in car engines and methane in gas ovens are examples of chemical changes Physical change does not alter the actual particles (molecules): it just separates them from one another: for example in freezing water the ice has the same number of molecules as the water did. Chemical change actually breaks the particles up: for example in electrolysis of water, the water molecules break up and hydrogen and oxygen molecules are formed). Physical changes just rearrange the particles without changing their nature whereas chemical changes break up the particles (molecules) and rearrange the atoms into new substances. But in both types of change there is no alteration to the actual number of each type of atom present.
49. Summarise the differences between the boiling and electrolysis of water as an example of the difference between physical and chemical change.

The 2 processes, boiling water and electrolysing water, clearly illustrates the difference between physical and chemical changes.Electrolysis of water is a chemical change as 2 new substances are produced whereas boiling water is a physical change, since it is only a change of state.Electrolysis produces 2 new substances (hydrogen and oxygen gases), whereas boiling does not produce any new substance (just converts liquid water to gaseous water)Electrolysis is difficult to reverse (need to mix the gases together and ignite them with a high temperature spark), whereas boiling is easily reversed (just cool the vapour and it goes back to liquid)Electrolysis requires much more energy than boiling (electrolysis requires between 20 and 30 kilojoules of electrical energy per gram of water decomposed (depending on conditions used), whereas boiling requires only 2.3 kilojoules per gram of water vaporised).
50. Identify light, heat and electricity as the common forms of energy that may be released or absorbed during the decomposition or synthesis of substances and identify examples of these changes occurring in everyday life.
Thermal decomposition
general decomposition reactions2H2O 2H2 + O2
2H2O2 2H2O + O2
H2CO3 H2O + CO2
CaCO3 CaO + CO2
2KClO3 2KCl + 3O2
The process by which heat breaks compounds down into simpler substances is known as thermal decomposition. In this process, which is a type of chemical change, the arrangement of particles in the compounds changes. The particles are rearranged to form different compounds or elements.The compound mercury (2) oxide, when heated strongly, decomposes into the elements mercury and oxygen
2HgO(s) 2Hg(l) + O2(g)
This reaction is a chemical change in which the actual substances present have been altered. The reaction has changed the nature and arrangement of the particles involved. The Hg2+ ions that were arranged with O2- ions in the solid HgO have changed to Hg atoms in liquid mercury. Similarly, the O2- ions that were in the HgO solid have changed to oxygen atoms covalently bonded in O2 molecules. No atoms have been destroyed or formed in this process. The form of the atoms, such as Hg atoms or Hg2+ ions, have changed but the atoms have been conserved.

Another compound, copper (2) carbonate, decomposes when strongly heated to form different compounds- copper (2) oxide and carbon dioxide.
CuCO3(s) CuO(s) + CO2(g)
Again in this process the nature and arrangement of particles has changed. Solid CuCO3 contains Cu2+ ions and CO3
2- ions. After the reaction, the Cu2+ ions remain but are now arranged with O2- ions in solid CuO, and CO2 gas molecules have been produced.
In baking, the thermal decomposition of bicarb soda (sodium hydrogencarbonate) to produce carbon dioxide gas is used to make cakes rise when they cook.
2NaHCO3(s) Na2CO3(s) + CO2(g) + H2O(g)Similarly, lime (calcium oxide), which is used as a treatment for acidic soils, is produced by the thermal decomposition of limestone (calcium carbonate).
CaCO3(s) CaO(s) + CO2(g)
Decomposition by electrical energy and light energyOther forms of energy such as electricity and light may also bring about the decomposition of compounds. Water can be decomposed into the elements hydrogen and oxygen if an electric current is passed through it. This is called electrolysis. The reaction is:
2H2O(l) 2H2(g) + O2(g)
Many other substances can be decomposed in this way. In fact, it was the process of electrolysis that greatly increased our ability to extract metals from their ores. Metals such as aluminium and sodium can only be extracted from their ores in this way.
Light energy can also cause the decomposition of some compounds. For example, silver salts such as silver chloride decompose when exposed to light, to produce silver metal. The use of silver bromide in black and white photographic film depends on this decomposition reaction. In this process, light causes the following decomposition reaction to occur:
AgBr(s) Ag(s) + 1 Br2
2
The Ag+ ions are converted to Ag atoms. These form a ‘latent’ image, which is enhanced when the photographic film is developed.
Many dyes and pharmaceutical products are also ‘light sensitive’ and will undergo decomposition if exposed to light. It is for this reason that many of these substances are stored in dark glass or opaque containers.

Synthesis of chemical substances
General synthesis reactions
2Na + 2Cl 2NaClS + O2 SO2
4Fe + 3O2 2Fe2O3
CO2 + H2O H2CO3
Many of the products we use, some of the foods we eat and most of the medicines we take are products of chemical synthesis. Synthesis is the process of forming a compound from elements or from other compounds. This process involves chemical change and usually leads to the formation of a more complex substance. Elements cannot be synthesised in chemical processes. They are extracted from naturally occurring ores.Initially many of the compounds synthesised by humans were produced because a previous naturally occurring source was diminishing or limited. An example of this is the synthesis of ammonia. In the 19th and early 20th centuries, farmers depended on animal manure and naturally occurring sodium nitrate deposits as sources of nitrogenous fertilisers. The need for fertiliser increased, and this lead to the development of the Haber process, in which hydrogen as and nitrogen gas are combined to form ammonia.N2(g) + 3H2(g) 2NH3(g)
While the overall reaction in the Haber process releases energy, fairly high temperatures are required to break the chemical bond in nitrogen and hydrogen so the reaction can take place.The ammonia is often reacted with sulfuric acid or nitric acid to form ammonium sulfate or ammonium nitrate, which can be used as fertilisers.
2NH3(g) + H2SO4(aq) (NH4)2SO4(aq)
Another example in which chemical synthesis has replaced natural resources is the dyeing industry. Initially all dyestuffs were natural products obtained primarily from vegetable sources. Indigo, for example, was derived from plants and Tyrian purple from a particular mollusc. Work by chemists in the 19th century, notably Henry Perkins, developed method of synthesising a wide range of coloured dyestuffs using chemicals isolated from coal. This greatly increased the availability of coloured dyes for fabrics and clothing.The plastics industry is another major industry that synthesises an extraordinary array of useful chemical substances starting with chemicals derived from petroleum. These include polyethylene, polystyrene, PVC, PET, Perspex, Teflon, nylon, Kevlar, and many others.In these various synthesis processes, energy often has to be provided in order to allow for the rearrangement of atoms in the reacting compounds. Whether a particular reaction absorbs or releases energy depends on the relative bonding strengths within the reactants and products. However, even if energy is released in a reaction, the reactants often need

to have energy supplies, usually in the form of heat or light, in order for the initial bond rearrangement to take place.
51. Explain that the amount of energy needed to separate atoms in a compound is an indication of the strength of the attraction, or bond, between them.
Decomposing a compound into elements requires a large input of energy because it is necessary to overcome the strong chemical bonds holding the atoms together in compounds. There are strong electrostatic attractions holding ions together in ionic compounds and strong covalent bonds holding atoms together in covalent molecules and in covalent lattices.The stronger the chemical bonding in a compound is, the more energy is required to break the compound into elements. Alternatively, the stronger the chemical bonding in a compound the more energy that is released when the compound is formed from its elements. When liquid water is heated, a physical change, intermolecular forces are broken. This requires little energy because the intermolecular forces are weak forces. The chemical process in which electricity is used to decompose liquid water into hydrogen and oxygen involves the breaking of strong covalent bonds between hydrogen and oxygen, as a result large amounts of energy are required to do so.
52. Identify differences between physical and chemical properties of elements, compounds and mixtures.
Physical propertiesOdour, colour, taste, lustre, hardness, density, mechanical strength, malleability (the ability to be beaten into sheets), ductility (the ability to be drawn into wires), electrical conductivity, thermal conductivity, melting point, boiling point, solubilityPhysical properties are those that can be determined without changing the chemical composition of the substance of the substance. For example, the physical properties of iron include the following: grey solid, metallic lustre, fairly soft when pure, malleable, ductile, good electrical and thermal conductor, high melting point (1535oC).
Chemical propertiesReactions with oxygen, water, acids, bases; specific reactions with other substancesChemical properties are those that relate to the ability of a substance to form new substances. Chemical properties include changes that occur when a substance breaks down or reacts with other substances during a chemical reaction. The chemical properties of iron include the following:
53. It reacts slowly with moist air to form rust.54. If finely divided, it will burn in oxygen when heated.55. It reacts with steam and dilute acids to form hydrogen.
56. Describe the physical properties used to classify compounds as ionic or covalent molecular or covalent network.

Ionic SubstancesHigh melting point, non- conducting in the solid state but conductivity in the liquid state.
Covalent Molecular SubstancesLow melting point, non- conducting in the solid and liquid states
Covalent Network SubstancesHigh melting point, non- conducting in the solid and liquid states
Metallic substancesHigh melting point, high conductivity in the solid and liquid state
Ionic compounds57. Hard and brittle.58. Non- conductors of electricity in the solid state, but good conductors when molten
or in aqueous solution.59. High melting and boiling points.
Covalent Molecular Substances60. They have low melting and boiling points, many are liquids or gases at room
temperature.61. They are non- conductors of electricity in both the solid and liquid states.62. They form solids that are generally quite soft and often have a waxy appearance.
Covalent Network Substances63. Very high melting and boiling points. 64. Non- conductors of electricity in the solid and liquid state. 65. Extremely hard and brittle.66. Insoluble in water and most other solvents.
Metals67. Relatively high densities (although lithium, sodium and potassium are less dense
than water)68. Good conductors of heat and electricity69. Malleable (can be beaten into sheets) and ductile (can be drawn into wires)70. Have a shiny surface when freshly cut or cleaned (lustrous)71. Relatively high melting points (although mercury and gallium have quite low
melting points).72. Solids at room temperature with the exception of mercury
73. Distinguish between metallic, ionic and covalent bonds.
See definitions: Ionic, Covalent

Metallic bonding- a 3- dimensional orderly array of positive ions held together by a mobile ‘sea’ of delocalised electrons. The valence electrons break away from their atoms, leaving behind positive ions. These free electrons, called delocalised because they no longer belong to particular atoms, move randomly through the lattice and, by being shared by numerous positive ions, provide the chemical bonding which holds the crystal together.
74. Describe metals as 3- dimensional lattices of ions in a sea of electrons.
The properties of metals arise because a metal consists of an orderly 3- dimensional array of positive ions held together by a mobile ‘sea’ of delocalised electrons. The valence electrons break away from their atoms, leaving behind positive ions. This is consistent with the fact that metals have small ionisation energies and low electronegativity, and hence the valence electrons are not strongly held. These free electrons, called delocalised electrons (because they no longer belong to particular atoms, therefore called non- directional bonding), move randomly through the lattice and, by being shared by numerous positive ions, provide the chemical bonding which holds the crystal together. It is the ability of these delocalised electrons to move freely through the lattice which causes metals to be good conductors of electricity. The delocalised electrons are also responsible for the transmission of heat energy in metals. The mobile electrons acquire heat energy from the heat source and rapidly transfer it to the cooler parts of the lattice. Metals can be bent, rolled into sheets, and drawn into rods and wires. These processes are possible because when the orderly array of positive ions is sheared, the mobile electrons are able to adjust to the new arrangement of positive ions and again provide the ‘glue’ to hold the whole assembly together. It is also because there is limited resistance to the movement of ions with respect to one another.The high densities indicate that in metals the cations are packed tightly together.
75. Describe ionic compounds in terms of repeating 3- dimensional lattices of ions.
X- Ray diffraction evidence suggests that the ions in an ionic solid are arranged in a regular 3- dimensional lattice. In the lattice of sodium chloride, each positive sodium ion is surrounded by 6 negative chloride ions and each negative chloride ions is surrounded by 6 positive sodium ions. In the solid compound, the position of the ions is fixed, and apart from vibration about these fixed positions no other movement of the ion occurs.
76. Explain why the formula for an ionic compound is an empirical formula.
For such ionic compounds, the formulae (for example NaCl, CaF2) specify the ratios in which the atoms (or ions) are present, not the composition of discrete molecules. Such formulae that give the ratio by atoms of elements in a compound rather then the actual numbers of atoms in a molecule are called empirical formulae. Formulae for ionic compounds are therefore always empirical formulae (because there are no molecules).

77. Identify common elements that exist as molecules or as covalent lattices.
Several elements exist as covalent molecules:1. H2, F2, Cl2, O2 and N2, are diatomic gases2. Br2 is a diatomic liquid while I2 is a diatomic solid3. Phosphorous and sulphur exist as covalent P4 and S8 covalent molecules
respectively.The noble gases (He, Ne, Ar, Kr, Xe, Rn) exist as monatomic molecules, that is without any chemical bonding at all.
Some other elements exist as covalent lattices:78. Carbon exists as diamond which is a 3- dimensional lattice and as graphite which
is a 2- dimensional lattice. SiO2, WC (Tungsten Carbide), SiC (Silicon Carbide).79. The semi- metals B, Si, Ge, As, Sb, and Te closely approximate to covalent
lattices though their bonding electrons are not as firmly localised as in diamond.
80. Explain the relationship between the properties of conductivity and hardness and the structure of ionic, covalent molecular and covalent network structures.
Ionic SubstancesThe strong electrostatic attraction between pairs of ions makes ionic substances hard. If the orderly array of ions is disturbed by applying a strong force, then ions of the same charge come close together. They then repel each other and this causes the crystal to shatter. This means that ionic crystals are brittle.The high melting and boiling points of ionic substances are also due to the strength of the electrostatic attractive forces between the oppositely charged ions.Solid ionic compounds do not conduct electricity because in the solid the ions are tightly bound into an orderly array and so are unable to move towards a charged electrode. When ionic substances melt however, the orderly arrangement of ions is broken up and the ions can move about relatively freely. They can then migrate towards a charged electrode; so molten ionic substances conduct electricity but not as well as metals.
Covalent MolecularCovalent molecular substances only have weak forces holding the molecules to one another and so they have low melting and boiling points. Because these weak intermolecular forces are easily overcome, it is easy to distort a solid covalent molecular substance. This means that such solids are soft. However, a large amount of energy is required to separate molecules into individual atoms. This indicates that there must be strong bonding forces. The non- conductivity of covalent molecular substances is due to the lack of mobile charged species in the solid and liquid states. There are no ions or free- moving electrons capable of conducting an electric current. Covalent molecular substances cannot conduct electricity either as pure substances or in solution (for example iodine, sucrose or urea in water). However some covalent substances when mixed with water actually react with the water and form ions: these solutions do conduct electricity.

Covalent Network SolidsThe key to their properties lies in the 3- dimensional network of strong covalent bonds between the atoms that hold the lattice together. The high melting and boiling points and the extreme hardness of covalent network substances suggest that the atoms in these solids are joined by very strong covalent bonds. These strong covalent bonds produce rigid 3- dimensional network structures, as seen in silicon carbide and silicon dioxide.The non- conductivity of covalent network substances is due to the lack of mobile charged species in the solid and liquid states. There are no free- moving electrons or charged ions capable of conducting an electric current (graphite is an exception).
METALS

81. Outline and examine some uses of different metals through history, including contemporary uses, as uncombined metals or as alloys
The first metals used by humans were those that were found in their elemental form, that is, uncombined with other elements. Gold, Silver and copper can be found as almost pure elements in various part of the world and were initially used by humans to make jewellery, ornaments and tools. The ability of these metals to be beaten and bent into various shapes and their relative scarcity made them prized possessions.
Early uses of copperInitially copper was used to make ornaments, tools, weapons and cooking implements. The Egyptians made and wore copper beads and used copper pipes to convey water. The excellent thermal conductivity of copper made it ideal as a cooking pot, a use that continues to the present day.One of the disadvantages of copper, regarding its use in weapons and tools, is that it is a rather soft metal. The chance discovery that a small amount of tin added to the copper increased the hardness of the metal was a major breakthrough.
Bronze: an alloy of copperThe deliberate addition of tin to copper to produce a much harder metal alloy, bronze, was of such importance that this stage of human cultural development is called the Bronze age (3000- 1000 BC). Bronze had many advantages over copper. Bronze cutting tools, such as axes and knives, maintain good cutting edges and are easily resharpened. Bronze shields and armour were much stronger that any other material available at the time. This was of strategic significance to nations waging war on their neighbours. Bronze also casts extremely well and is very durable. Statues many thousands of years old are evidence of the durability of bronze and the metallurgical skills of ancient civilisations.
Contemporary uses of copperContemporary uses of copper are mostly related to its excellent electrical and thermal conductivity, resistance to corrosion and ability to form a huge range of alloys. Copper is second only to silver as an electrical conductor and is used extensively in electrical cables and wiring, appliances, electrical generators and motors. Copper

pipes, tanks and hot water systems are used for plumbing because copper does not corrode in hot water.Copper piping is also used in air- conditioning units and heat exchangers such as car radiators because it loses heat quickly. Copper’s ability to form a wide range of alloys (more than one thousand different alloys have been formed) is one of the main reasons that copper remains one of the most important metals today.
The use of iron through historyThe iron age began around 1000BC. During this period, iron replaced Bronze in many applications, particularly the manufacture of tools and weapons. Pure iron is, however, susceptible to corrosion (rusting) and is relatively soft. It is likely that artisans discovered by accident that iron contaminated with carbon forms an alloy (steel) that is more corrosion resistant and harder than pure iron. The further discovery that the steel becomes even harder and keeps a better edge by heating it to a moderate temperature and then dunking it is cold water meant that bronze was eventually replaced by iron in many applications.Although carbon steels (iron- carbon alloys) were probably first made about 1000BC, the large- scale production of steel did not begin until the middle of the nineteenth century. Today there are many types of steel that have various uses, particularly in building construction and in the manufacture of cars, machinery and household appliances. Iron remains the most abundant, useful and important of all metals.
Contemporary uses of metalsCopper and iron are still widely used today. However, other metals such as aluminium and titanium are now also of considerable importance.
AluminiumDespite being the most abundant metal in the Earth’s crust and possessing some remarkable useful properties, aluminium was not used extensively until the beginning of the twentieth century. The reason for this is that aluminium was extremely difficult and expensive to extract from it ore, bauxite, which contains the mineral gibbsite, Al2O3.3H2O, together with various impurities.After a commercially viable method of extracting Aluminium had been developed, the use of aluminium increased dramatically.Due to its low density and its resistance to corrosion, aluminium has displaced steel in many commercial and industrial situations. For example, aluminium is used widely in building construction as roofing, window frames, appliance trim and decorative furniture. It is also used in the manufacture of a range of domestic utensils such as saucepans, frying pans, drink cans and cooking foil.Aluminium is also an excellent electrical and thermal conductor and is highly reflective, making it useful in such diverse applications as electrical transmission lines, telescope reflectors, food packaging and saucepans.Aluminium can be strengthened by the addition of small amounts of other metals such as titanium to produce many alloys that are valued for their low density and strength. As a result they are extensively used in spacecraft, aircraft and boat construction

TitaniumAlthough titanium was discovered in 1791, it was not until 1910 that the pure metal was isolated.Titanium is the 9th most abundant element in the Earth’s crust, occurring primarily in the minerals rutile and ilmenite. Pure titanium is a lustrous solid, similar in appearance to stainless steel. It melts at high temperatures, has a low density and great strength and is very resistant to corrosion. Alloys of titanium are very strong and are used in situations where lightweight strength and resistance to high temperatures are required, such as in jet engine components, aircraft, spacecraft and missiles.Titanium alloys are biocompatible and therefore can be used for surgical implants such as artificial knee and hip joints.Because titanium is so resistant to corrosion, it is used in marine environments as propellers and other ship parts and in chemical factories where corrosive acids are used. Titanium is also added to other metals such as aluminium, iron, manganese and molybdenum to improve their strength.
Metal Properties Uses
Copper Excellent thermal and electrical conductor, malleable and ductile, low reactivity, readily forms alloys
Electrical cables and wiring, radiators, refrigeration systems, water pipes, alloys including bronz (casting) and brass (fittings and fixtures)
Iron Soft, malleable, magnetic, good thermal and electrical conductor, fairly reactive, readily forms alloys
Due to its susceptibility to corrosion it is usually converted to steel, which is used in buildings and bridges, automobiles, machinery and appliances
Aluminium Low density, relatively soft when pure, excellent thermal and electrical conductor, malleable and ductile, good reflector of heat and light, readily forms alloys
Saucepans, frying pans, drink pan, cooking foil, food packaging, roofing, window frames, appliance trim, decorative furniture, electrical cables, aircraft and boat construction
Titanium Great strength, high melting point, low density, low reactivity, readily forms alloys
In lightweight, high- strength alloys used in high – temperature environments, e.g. spacecraft and aircraft, pipes and linings for vats where acids are used
Chromium Shiny silver appearance, resists corrosion, readily forms alloys
Plating other metals, as an additive in steel alloys, e.g. stainless steel
Cobalt Magnetic, readily forms alloys In alloys such as alnico to manufacture permanent magnets

Nickel Magnetic, readily forms alloys As an additive in steel alloys, invar (Fe, Ni) used in scientific instruments, as an alloy (Ni, Cu) in making coins, nichrome (Ni, Cr) used in electrical heating elements
Zinc Fairly reactive but forms protective oxide layer, readily forms alloys
In galvanising iron, in the outer casing and negative electrode of dry cells, alloy (brass) used in fittings and fixtures
Gold Shiny gold appearance, excellent thermal and electrical conductor, unreactive, readily forms alloys
Electrical connections, jewellery, monetary standard, dentistry
82. Describe the use of common alloys including steel, brass and solder and explain how these relate to their properties
Alloy Composition Properties UsesBronze 90% Cu, 10%Sn Strong, resists wear and
corrosion, easily cast, sonorous
Church bells, statues, bearings
Brass 65% Cu, 35% Zn Resists corrosion, ductile, easy to machine, polishes well
Doorknobs, screws
Cupronickel 75% Cu, 25% Ni Hard- wearing, attractive silver colour
‘Silver’ coins, for example, 10c piece
Stainless steel
74% Fe, 18% Cr, 8% Ni
Resists corrosion Sinks, cutlery
Mild Steel 99.8% Fe, 0.2% C Hard but easily worked Nails, cables and chainsSolder 33% Sn, 67% Pb Low melting point Joining pipes and wires
Duralium 96% Al, 4%Cu Low density but very strong
Aircraft bodies
Pewter Old: 70% Pb, 30% Sn.Modern: 91% Sn, 7.5% Sb, 1.5% Cu
Easily worked, durable. Modern pewter attractive silver colour.
Drinking jugs, ornaments
Jewellery gold (14 carat)
58% Au, 42% other, for example, Ag, Cu, Pd, Pt
Easily worked, harder than pure gold, unreactive, attractive gold colour
Jewellery, ornaments
Sterling silver
92% Ag, 8% Cu Easily worked, harder than pure silver, unreactive, attractive
Jewellery, ornaments

silver colourDental amalgam
60% Ag, 28% Sn, 12% Cu, 0.05% Pt dissolved in Hg. Other metals: Hg ratio1:0.73 (42% Hg)
Easily worked Fillings for teeth
SteelIron, in various forms of steel, is used extensively in building construction and the manufacture of cars and other machinery. These steels usually contain small amounts of carbon alloyed with iron. The properties of carbon steels can be changed by altering the amounts of carbon added to the iron. Increasing the proportion of carbon increases the strength and hardness of the steel produced. They can be further changed by ‘working’ the steel in forging and rolling processes, and by heat treatment such as annealing, quenching and tempering.Some specialist steels contain small amounts of other metals. For example, stainless steel used in cutlery and sinks consists of iron alloyed with chromium and nickel. Some steels have other metals added to form ‘alloy steels’ with special properties.
Type Element Alloying With Iron
Major Properties Uses
Carbon SteelsMild steel <0.2% C Ductile and
malleableNails, chains, cables, machinery
Structural steel 0.2- 0.5% C Hard, strong Girders, vehicles, railsTool steel 0.9- 1.5% C Very hard, strong Tool blades, cutleryAlloy SteelsStainless 10- 25% Cr, 4- 22%
NiCorrosion resistant
Cutlery, sinks, hospital items
Chrome 2-5% Cr Very hard, tough Armour plate, gears
Chrome- vanadium
3-10% Cr, 1-5% V Great tensile strength
Springs, car parts
Manganese 10-15% Mn Very hard, abrasion resistant
Safes, crushers, mechanical shovels
Molybdenum 5% Mo Strong, heat resistant
Drive shafts, gears
Tungsten 12-20% W, 2-5% Cr, 1-3% V
Hard at high temperatures
Cutting and grinding tools
Silicon 2-5% Si Easily magnetised and demagnetised
Electromagnets, transformers

Alnico 6-12% Al, 14-20% Ni, 2-35% Co
Retains magnetism
Permanent magnets
Invar 36% Ni Low rate of expansion
Pendulums, surveyor’s tapes
SolderSolder is an alloy of lead and tin. It has a melting point lower than either of these pure metals. Solder is a metal filler used to join two pieces of metal together. The solder is melted and allowed to fill the space between the pieces to be joined, and as it cools it solidifies, bonding the 2 pieces together. Soldering is used extensively in the electronics industry.
BrassBrass is an alloy consisting mostly of copper and zinc, although small amounts of other elements such as lead, tin and aluminium may be present. Brass is resistant to corrosion and therefore brass fasteners (screws, bolts, nails and so on) may be used in situation where other metals would rust. Brass also has an attractive finish and is used for decorative elements such as doorknobs and knockers.
83. Explain why energy input is necessary to extract a metal from its ore
To extract a metal from its mineral or ore, a large input of energy s required. This energy is required to break and rearrange the strong chemical bonds within the compound. In general, the ease of extraction of a metal from a mineral is directly related to the strength of the bonds within the metal compound and to its position in the activity series. For example the reaction between copper (1) sulfide and copper (1) oxide to produce copper and sulphur dioxide requires a significant input of energy because high temperatures are needed to maintain this reaction.Energy is needed to mine the ore and to purify or concentrate it. Energy is required to maintain the high temperatures needed to make the extraction reaction go, and energy is needed to purify the raw metal or to form it into useful alloys. Overall a large amount of energy is needed to extract a metal from its ore.Also, vast amounts of energy are required to isolate pure Aluminium oxide, alumina from bauxite and then to extract aluminium from alumina. The refining of bauxite to produce Alumina uses about 15000MJ of energy per tonne of alumina produces. Smelting this alumina requires an additional 50000MJ per tonne of aluminium produced. The huge amount of energy requirements are largely due to the electrolytic reduction of Alumina to Aluminium in the Hall- Heroult process. Since little energy is required to separate copper from its ore, this indicates its metal compound bonds are not very strong, resultantly it was one of the first elements to be extracted.
84. Identify why there are more metals available for people to use now than there were 200 years ago
Aluminium is a more reactive metal then iron and cannot be extracted by chemical

reduction. The extraction of metals higher on the activity series, such as Aluminium, did not take place until the nineteenth century. This is because more reactive metals can only be extracted by electrolysis. As a result of it reactivity, Aluminium can only be extracted from its ores using the more difficult and expensive method of electrolytic reduction. This method of production of Aluminium had only been developed early in the twentieth century. Therefore the use of Aluminium does not appear until quite recently in Human history.Mineral resources are also being extended in several ways. First, new ore bodies are being discovered, often using advanced exploration techniques in geophysics and remote sensing. Second, improving technology is increasing our ability to access some ores, such as those that occur at considerable depth in the Earth’s crust or under the oceans. Third, our ability to extract metals from lower- grade ores is improving. Fourth, as we learn more about factors affecting the rate of corrosion of metals we are better able to protect metal items and structures from corrosion. An increasingly important option in extending our metal resources, and reducing energy use, is to recycle metals from objects that no longer serve their original purpose.
85. Describe observable changes when metals react with dilute acid, water and oxygen
Reactions of metals with oxygenAll metals except silver, platinum and gold react with oxygen to form oxides:
86. Li, Na, K, Ca, Ba, react rapidly at room temperature, tarnish quickly when exposed to air and must therefore be stored in liquid paraffin oil.
87. Mg, Al, Fe, Zn react slowly at room temperature but burn vigorously if heated in air or pure oxygen.
88. Sn, Pb, Cu react slowly and then only if heated.
All the oxides formed are ionic compoundsThose metals which burn in air or oxygen form crystalline white solids which have none of the physical properties of the original metal (lustre, strength, malleability, conductivity). When metals slowly react at room temperature, they lose their shiny lustrous appearance. Some, such as Al and Zn, become coated with a dull layer of tightly adhering oxide which prevents further reaction.Others such as iron form a powdery surface layer of oxide which does not impede further reaction. When copper is heated in air it forms a black surface layer of copper oxide.
Reaction of Metals with waterSome metals react with water or steam while others do not:
89. Li, Na, K, Ca, Ba react with water at room temperature90. Mg, Al, Zn, Fe react with steam at elevated temperatures91. Sn, Pb, Cu, Ag, Au, Pt do not react at all
When reaction occurs with water the products are hydrogen gas and a metal hydroxide.

The formation of hydrogen in this reaction can be confirmed by collecting a sample of the gas produced and introducing a lighted splint. A small explosion or ‘pop’ confirms the presence of hydrogen. An acid- base indicator such as phenolphthalein or litmus can be used to confirm the production of hydroxide ions.When pieces of lithium, calcium and barium are dropped into water, bubbles of colourless gas form: with lithium and barium a colourless solution of lithium or barium hydroxide results, but with calcium a suspension of insoluble white calcium hydroxide forms. Sodium and potassium react very vigorously (often with an explosion as the hydrogen ignites in air)When a piece of freshly cleaned magnesium ribbon is held in the mouth of a flask of vigorously boiling water, a white deposit (of magnesium oxide) forms on the ribbon as the magnesium reacts with steam. Steam needs to be heated significantly above 100oC for the reaction with Aluminium, iron and zinc to occur. With steam the product is oxide, not hydroxide.
Reactions of metals with acidsA number of metals that do not react with water will react with dilute acids. Metals such as magnesium, zinc and iron react quite readily with cold dilute hydrochloric or sulfuric acid to produce hydrogen gas and salts. In fact, the laboratory preparation of hydrogen usually employs the reaction of zinc with dilute hydrochloric or sulfuric acid. Metals more reactive than magnesium, such as Sodium, potassium, and calcium, react explosively with dilute hydrochloric acid and sulfuric acids. Less reactive metals such as coper, silver and gold do not react with these acids. The reaction is also characterised by a fizzying sound and bubbles being formed. Zinc, for example, reacts with dilute hydrochloric acid to form bubbles of colourless hydrogen gas and a clear solution of zinc chloride.
92. Describe and justify the criteria used to place metals into an order of activity based on their ease of reaction with oxygen, water and dilute acids
The reactivity of metals with oxygen, water and dilute acids can be used to draw up a list of metals in order of decreasing reactivity. This list is called an activity series. Some common metals are arranged according to their ability to displace hydrogen from dilute acids and water.
{Na, K} > {Li, Ca, Ba} > {Mg, Al, Fe, Zn} > {Sn, Pb} > Cu > {Ag, Au, Pt}
This is an activity series for the common elementsSome of these metals appear to have similar reactivities with oxygen, water and acids and so are grouped as metals with similar reactivities. This is as far as we can go based upon reactions with oxygen, water and dilute acids, but to separate the metals that have equal reactivities we need to use either displacement reactions or voltage measurements from galvanic cells (devices which generate electricity from chemical reactions). Use of such reactions or measurements to separate metals rated equally in the above list gives
K, Na, Li, Ba, Ca, Mg, Al, Zn, Fe, Sn, Pb, Cu, Ag, Pt, Au

The reactions under consideration involve the metal atoms losing electrons to become metal ions. The reactivity series then lists the metals in order of decreasing ease of losing electrons: metals to the left lose electrons more easily than metals to the right. Loss of electrons means oxidation, so the activity series lists the metals in order of decreasing ease of oxidation. Conversely, the activity series lists the metals in order of increasing ease of reduction of the metal ions.
93. Identify the reaction of metals with acids as requiring the transfer of electrons
During the reaction between a metal and an acid, the metal dissolves as it losses electrons and forms positively charged ions. Hydrogen ions from the acid gain electrons to form hydrogen gas. As this reaction involves the transfer of electrons it is an oxidation- reduction reaction. These two simultaneous reactions can be represented by the following equations: Oxidation is loss of electrons, and reduction is gain of electrons.
Zn(s) Zn2+(aq) + 2e- Zn(s) + 2HCl(aq) ZnCl2 (aq) + H2 (g)
2H+(aq) + 2e- H2 (g)
Mg (s) Mg2+(aq) + 2e1- Mg(s) + H2SO4 (aq) MgSO4 (aq) + H2 (g)
2H1+(aq) + 2e1- H2 (g)
94. Outline examples of the selection of metals for different purposes based on their reactivity, with a particular emphasis on current developments in the use of metals
The uses of a particular metal are determined not only by its physical properties but also by its reactivity. Gold, Silver and platinum are quite unreactive and undergo little or no corrosion. Because these metals do not readily tarnish, they retain their shiny lustre and make attractive jewellery. Gold is the least reactive of all metals. This has been known since ancient times and throughout history it has been used to make jewellery and fine ornaments. Because gold is an excellent conductor of electricity and does not tarnish, it is also widely used in electrical connections in computer and electronic circuits. Gold also has several applications in the space industry such as in the plating of the umbilical cord that joins astronauts to their spacecraft. This use is made possible because gold is and excellent reflector of infra- red radiation and is chemically inert. It would have to be chemically inert in order to be a good reflector, otherwise it would tarnish.
Magnesium is a highly reactive metal and some of its uses are a result of this reactivity. For example, magnesium is used in the cathodic protection of less reactive metals to protect them from corrosion. Steel ships and wharves may have large blocks of magnesium attached to them. Since magnesium is more reactive then the iron in the steel it will corrode first, thus protecting the ship and wharf from corrosion. For this reason the magnesium is called a sacrificial anode.When magnesium burns in oxygen it produces a bright white light. Because of the intensity of this light, magnesium was used in photographic flashbulbs and it continues to

be used in modern fireworks.
Owing to its high reactivity, the uses of calcium are restricted to situations where its reactivity can be used to advantage. Calcium is added to some steels to remove any remaining traces of oxygen, sulphur, and phosphorous. It is also used in electronic vacuum tubes because it combines with any traces of oxygen that remain, producing a better vacuum.
Zinc is used extensively in galvanised iron production. The galvanised iron is produced by dipping iron into molten zinc. The zinc serves two purposes. First, the zinc forms a protective coating for the iron, excluding oxygen and therefore protecting the iron from rusting. The zinc reacts with the air to form an impervious layer that protects it from corrosion. The second way in which the zinc protects the iron from corrosion is that, in the event that the zinc coating of the iron is damaged, the zinc acts as a sacrificial anode and corrodes in preference to the iron.
The reactivity of zinc also makes it suitable for use in batteries such as dry cells (torch batteries) and button cells (in watched). In these cells the zinc is oxidised and the electrons it loses travel through an external circuit, producing an electric current.
Tin and chromium are used widely to coat a base metal, usually steel, in many applications. This use is due to the low reactivity of these metals together with their shiny appearance. Tin cans commonly used as packaging for foods such as soups, baked beans and canned fruit are actually steel cans coated in tin. The bumper bars of many older cars and so- called chrome trimmings are usually steel coated with chromium metal. Both tin and chromium form an impervious oxide layer that protects the underlying metal from corrosion.
Copper, aluminium and titanium are also widely used because of their low reactivity and/ or their resistance to corrosion. Copper is used extensively in the plumbing industry because of its ability to resist corrosion. Unlike the cheaper alternate, iron, copper does not react with hot water and is ideal for hot water tanks and pipes. Aluminium, because of its ability to form a protective oxide layer and it s light weight, is extensively used in the building industry. Titanium is used in holding tanks and pipes in desalination plants, which convert sea water into fresh water, and factories producing or using acids. The ability of titanium to resist corrosion makes it an ideal metal in these corrosive environments.
95. Outline the relationship between the relative activities of metals and their positions on the Periodic Table.
A comparison of the activity series for metals with the position of these metals in the periodic table reveals some interesting relationships. The activity series shows that Group 1 metals are the most reactive followed by Group 2 metals. Group 3 (Al) comes next in reactivity followed by some transition metals (Zn, Fe), then the metals of Group 4 (Sn, Pb). At the end of the series are more transition metals (Cu, Ag, Pt, Au). The activity

series also shows that in Groups 1 and 2 reactivity increases from top to bottom (Li to k, Mg to Ba).
96. Identify the importance of first ionisation energy in determining the relative reactivity of metals
The relative ease with which a metal loses its valence electrons is a major factor affecting its reactivity. Very reactive metals such as potassium and sodium lose their valence electrons relatively easily. Less reactive metals such as copper do not lose their valence electrons as readily, and gold and silver rarely lose their electrons at all. Ionisation energy is a measure of the energy needed to remove an electron from the electrostatic attractive force of the positively charged nucleus. The ionisation energy of an atom or ion is defined as the amount of energy required to remove the most loosely bound electron from the atom or ion in the gaseous state. The energy required to remove the first electron from an atom is called the first ionisation energy.In general, reactive metals tend to have low ionisation energies and less reactive metals have higher ionisation energies. The reactivity of metals increase as their ionisation energy decreases.
M(g) + energy M+(g) + e-
Gaseous atom gaseous ion
97. Identify an appropriate model that has been developed to describe atomic structure
PREVIOUS MODULE
98. Outline the history of the development of the Periodic Table including its origin, the original data used to construct it and the predictions made after its construction
Early in the nineteenth century as more and more elements became known, attempts were made to see patterns in their properties. In 1829 with about 40 elements known, the German chemist, Dobereiner, drew attention to several groups of three elements (which he called triads) with very similar properties;
Lithium, sodium, potassiumCalcium, strontium, bariumChlorine, bromine, iodine
In 1864 with over 60 known elements, John Newlands, an Englishman, proposed a ‘law of octaves’: when the elements were arranged in order of increasing atomic weight, ‘the eighth element starting from a given one is a kind of repetition of the first like the eighth note in an octave of music’. His ‘law’ identified many similarities among the elements, but erroneously required similarities where none existed. Newlands law works to about calcium but then breaks down because the element after do not resemble those eight

places before them.
In 1869 Dmitri Mendeleeve, a Russian, and Lothar Meyer, a German, independently produced the forerunner of the modern periodic table. They arranged the elements in order of increasing atomic weight, and placed elements having similar properties under one another to obtain a table which illustrated what they called the periodic law:Properties of the elements vary periodically with their atomic weights.This table was far more successful than that of Newlands, primarily because Mendeleeve recognised that there were probably elements in existence which had not been discovered at that time. He left gasps in his table in order to place similar elements under one another and proposed that there were undiscovered elements to fill the gaps. And he went further; he predicted the properties of six such elements, three of which he called eka- silica, eka- boron and eka- aluminium (‘eka’ meaning ‘like’). Subsequently, germanium, scandium, and gallium were discovered with properties remarkably similar to his predictions.
As time passed and atomic weights became more accurately known, some discrepancies emerged in this periodic table. In a few cases, in order to fit similar elements under one another, it was necessary to invert the order of atomic weights (argon and potassium, tellurium and iodine, cobalt and nickel). This problem was resolved in 1914 when the British scientist, Henry Moseley, determined what we now call the atomic number of each of the elements. He proposed that it, rather than atomic weight, was the basic feature which determined properties. Moseley proposed a modified periodic law:Properties of the elements vary periodically with their atomic numbers.This law put argon and potassium, cobalt and nickel, and tellurium and iodine in their right orders. Once it was recognised that properties were dependant upon number of protons (atomic number) and hence upon numbers of electrons, tendencies towards relating the layout of the Periodic table to electron configuration developed, and so the current form of the table was gradually devised.The periodic table developed through attempts to classify the elements- to put similar ones into families or groups.
Properties of eka- silicon (Es) as predicted by Mendeleeve
Obsered properties of Germanium (Ge) discovered in 1886
Colour grey Colour grey
Atomic mass 72 Atomic mass 72.6
Density 5.5g cm-3 Density 5.4g cm-3
Oxide has formula EsO2 and density 4.7g
cm-3
Oxide has formula GeO2 and density 4.2g
cm-3
Chloride has formula EsCl4 and density
1.9g cm-3
Chloride has formula GeCl4 and density
1.9g cm-3
99. Explain the relationship between the position of elements in the Periodic Table, and:
100. Electrical conductivity

101. Ionisation energy 102. Atomic radius 103. Melting point 104. Boiling point 105. Combining power (valency) 106. Electronegativity 107. Reactivity
Trends in properties across periods- Moving across a period from left to right108. Moving from left to right across a period, there is a general decrease in
atomic radius. The radius decreases because the increasing positive charge of the nucleus attracts the outermost electrons, which are in the same energy level, closer to the nucleus.
109. There is an increase in ionisation energy across each period, reaching a maximum at a noble gas. The increase in ionisation energy across a period is due to each successive element having one more proton in its nucleus. The extra electron for successive elements in a period occurs in the same energy level as other valence electrons. There is therefore a gradual increase in the attractive force between the nucleus and the valence electrons, leading to an increase in ionisation energy.
110. The melting points and boiling points generally rise to the elements in group 4 and then decrease
111. There is an increase in electronegativity of the elements. The electronegativity of an atom is a numerical measure of the electron- attracting power of the atom.
112. The elements change from metals through semi- metals to non- metals113. The bonding in the elements varies from metallic to covalent network to
covalent molecular114. The electrical and thermal conductivities decrease115. There is a regular pattern in the combining power of the elements. The
pattern is of increasing (to group 4) and then decreasing combining power across each period. This pattern can be detected by examining the formulas of the compounds formed by these elements
Trends in properties down groups- Moving down a group from top to bottomElements in the same group of the periodic table have the same number of electrons in their valence or outermost energy level, and as a result have similar properties. However, there is usually a gradual change in properties going down a group. The trends that are evident moving down groups in the periodic table include:
116. An increase in atomic radius. This occurs because each new period adds another electron energy level or shell that is more distant from the nucleus than the previous energy level.
117. A decrease in first ionisation energy. This occurs because the valence or outer energy level electrons are further from the nucleus in each successive period.
118. A decrease in electronegativity. This occurs because the valence electrons

energy level is at a greater distance from the nucleus in each successive period119. An increase in metallic character. In group 4, for example, the elements
change from a typical non- metal, carbon, at the top of the group, through the semi- metals silicon and germanium, to the typical metals tin and lead.
Define the mole as the number of atoms in exactly 12g of carbon- 12 (Avogadro’s number)
A mole is defined as the amount of a substance that contains the same number of particles as there are atoms in exactly 12g of carbon-12. Chemists have determined that the number of atoms in 12g of carbon-12 is 6.022 x 1023. This number is called Avogadro’s number after the Italian scientist Amadeo Avogadro. One mole of any substance contains 6.022 x 1023 particles of that substance.
Compare mass changes in samples of metals when they combine with oxygen
Describe the contribution of Gay- Lussac to the understanding of gaseous reactions and apply this to an understanding of the mole concept
Experiments measuring the combining volumes of gases led to an improvement in our understanding of the formulas of substances and the development of the mole concept. The French chemist Joseph Gay- Lussac found that there was a simple relationship between the volumes of gases involved in chemical reactions. For example, in the reaction of hydrogen and oxygen gas to produce water, the relationship is as follows:
Hydrogen gas oxygen gas steam2H2 (g) + O2 (g) 2H2O (g)+
2L of + 1L of 2L of steamhydrogen oxygen
Similar simple ratios are found to exist for other reactions involving gases. An example is the reaction of hydrogen gas and chlorine gas to produce hydrogen chloride.
Hydrogen gas chlorine gas Hydrogen chloride gasH2 (g) + Cl2 (g) 2HCl (g)
1 volume of + 1 volume of 2 volumes of hydrogen hydrogen chlorine chloride
Gay- Lussac’s law of combining volumes can be stated as follows:‘the ration of the volumes of gases involved in a reaction, if measured at the same

temperature and pressure, are expressed by small, whole numbers.’
Recount Avogadro’s law and describe its importance in developing the mole concept
Gay Lussac’s law was difficult to interpret at that time because the molecular formulas for hydrogen, oxygen and water were not known with certainty. However, in 1811, Amadeo Avogadro proposed that both hydrogen and oxygen consisted of diatomic molecules and that in the reaction between them the molecules actually split apart and recombined to form water molecules containing two atoms of hydrogen and one of oxygen. In applying Avogadro’s hypothesis to the formation of hydrogen chloride we deduce that one molecule of hydrogen combines with one molecule of chlorine to form two molecules of hydrogen chloride. Consequently, a molecule of hydrogen must contain at least two atoms of hydrogen (because it contributes to two molecules of hydrogen chloride), and similarly a molecule of chlorine must also contain at least two atoms.Likewise, in the formation of water, two molecules of hydrogen combine with one molecule of oxygen to form two molecules of gaseous water. Because one molecule of oxygen contributes to two molecules of water, it must contain at least two atoms of oxygen. Two molecules of hydrogen (which connot be monatomic) reacting with one molecule of oxygen meant that water could not be HO, and so was most likely to be H2O.
In an attempt to show how the volume of a gas varied with the amount of gas present, Avogadro proposed the following hypothesis:
‘Equal volumes of all gases, measured at the same temperature and pressure, contain equal numbers of molecules’
For example, this means that in 2L of hydrogen gas there must be the same number of molecules as there are in 2L of oxygen gas. It also means that the greater the volume of gas, the greater the number of molecules it will contain. For example, in 2L of hydrogen gas there must be twice the number of molecules as there are in 1l of oxygen gas, provided the volumes are measured at the same temperature and pressure.
Distinguish between empirical formulae and molecular formulae
The empirical formula of a compound specifies the simplest whole- number ratio of the numbers of atoms or ions of each element in the compound. This contrasts with the molecular formula, which specifies the actual number of atoms of each element in a molecule. For the compound hydrogen peroxide, the molecular formula is H2O2. Each Hydrogen peroxide molecule contains 2 hydrogen atoms and 2 oxygen atoms bonded together. However, the empirical formula of hydrogen peroxide is HO. This represents the simplest whole- number ratio of the numbers of atoms of each element.In many compounds, such as water (H2O), ammonia (NH3) and carbon dioxide (CO2), the empirical and molecular formulas are the same. Where the empirical and molecular formulas are different, the molecular formula is always a multiple of the empirical formula.

It is possible for different compounds to have the same empirical formula but they have to have different molecular formulas. Acetylene and benzene are two compounds with the empirical formula CH. The molecular formulas for these compounds are C2H2 and C6H6 respectively. The empirical formula of a substance can be established experimentally. This is achieved by determining the percentage composition of the substance by chemical analysis. This is then used to calculate the empirical formula of the substance.
Define the terms mineral and ore with reference to economic and non- economic deposits of natural resources
Mineral- A mineral is a pure (or nearly pure) crystalline compound that occurs in the Earth’s crust. Minerals are termed ‘minerals’ based on the above criteria and not on the economics of a particular mine. Ore- if the mineral is present in sufficient quantity to make the mining and extraction of the metal economically viable, it is called an ore.
Describe the relationship between the commercial prices of common metals, their actual abundances and relative costs of production
Apart from their specific properties other factors that affect the commercial price and extent to which particular metals are used include:
120. The abundance of the metal in the ore deposit121. The cost of extraction of the metal122. The demand for the metal
Metals vary markedly in their abundance in the Earth’s crust. For example, some metals are relatively abundant, such as Aluminium (8%) and iron (5%) respectively. Other metals are less abundant, such as copper (0.005%) and lead (0.001%), while others are rarer still, such as mercury (0.000008%) and gold (0.0000005%). More important than the overall abundance of various metals in the earth’s crust is the occurrence of ore deposits that contain a sufficient proportion of a particular mineral to make the mining of the ore and extraction of the metal economical. For example, although zinc makes up only about 0.008% of the Earth’s crust, it occurs in ores containing minerals such as zinc blende in sufficient concentration to make the mining and extraction of the zinc economically viable.
The cost of mining an ore and metal extraction is another important factor that affects the use of metals. Despite being the most abundant metal in the Earth’s crust, aluminium was once so expensive to extract that its use was severely limited, even though it had very many useful properties. It was only after a commercially viable method of extracting Aluminium from bauxite was developed that its cost was reduced and aluminium could be widely used. Iron, on the other hand, has been comparatively cheap for a long period of time. This is partly because iron ores are usually of a high grade and are reasonably abundant. In addition, the iron can be extracted from its ore on a large scale and at reasonably low cost in blast furnaces that run continuously, thereby reducing production

costs.
It is worth pointing out that the economic viability of metal extraction can vary depending on factors such as current demand and price, and improvements in metallurgical processes. For example, the efficiency of gold extraction has increased markedly with the development of the modern carbon- in- pulp process. This process makes it possible to profitably extract gold from much poorer ores than was previously possible. This has led to the re- treatment of old mine dumps, known as tailings.
Titanium is a highly sought- after metal despite its relative rarity in ores. The particular properties that make titanium so useful include its extreme toughness and durability, and resistance to corrosion. As a result, titanium is quite expensive
Explain why ores are non- renewable resources
Metal ores are non- renewable resources. They were formed when the Earth was formed and there is no way of forming any more of them. While we are unlikely to use up all the known reserves of metal ores in the short term, we nevertheless should use them as sparingly as possible so as to make them last for as long as possible.
Describe the separation processes, chemical reactions and energy considerations involved in the extraction of copper from one of its ores
Copper is principally extracted from sulfide ores, particularly chalcopyrite (CuFeS2). The copper ore may be on the surface and easy to excavate, or may be underground and require more sophisticated methods of excavation. The crude ore from the mine often contains less than 0.5% copper by mass and must be milled before the metal can be extracted. Milling refers to the concentration or purification of an ore. Copper is concentrated using a method called froth flotation. In this process, air is bubbled through a suspension of the pulverised ore in water containing a flotation agent. The desired copper mineral particles adhere to rising bubbles and are skimmed off as froth, leaving the unwanted silicate minerals to settle out. In this way, the copper ore is separated from the gangue.The concentrated ore which is now about 15% copper, is then roasted in air, which converts the FeS to FeO but leaves the CuS unaffected.
2CuFeS2 (S) + 3O2 (g) 2CuS(s) + 2FeO(s) + 2SO2 (g)
the product is then heated at 1100oC with ground limestone, sand and additional concentrated ore. This converts the FeO to a molten slag and converts the copper(11) sulfide to copper(1) sulfide.
FeO(s) + SiO2 (s) FeSiO3 (l)
In the final smelting step, the copper(1) sulfide is roasted in air so that part of it is converted to copper(1) oxide.

2Cu2S(s) + 3O2 (g) 2Cu2O(s) + 2SO2 (g)
The two copper(1) compounds, copper(1) sulfide and copper (1) oxide, then react together to form copper metal and sulphur dioxide.
2Cu2O(s) + Cu2S(s) 6Cu(l) + SO2 (g)
This process requires a significant input of energy to maintain the temperatures required for this reaction.The smelting process produces crude copper of about 98% purity, with is called blister copper because of the bubbly appearance produced by escaping sulphur dioxide. To obtain the 99.9% purity required for electrical wiring, the blister copper is refined electrolytically.The impure blister copper is cast into slabs and made the anode (positive electrode) of an electrolytic cell. The cathode is a thin sheet of pure copper and the electrolyte is copper(11) sulfate solution, acidified with sulfuric acid.
The copper and reactive metal impurities such as zinc, iron and nickel present in the anode dissolve and enter the solution as ions. As the anode slab dissolves, the more inert metal impurities such as gold, silver and platinum fall to the bottom of the cell. In a finely divided state, they form an ‘anode slime’, which is a profitable source of these metals.
A small voltage is used so that pure copper deposits at the cathode (negative electrode). The ions of the more reactive metals remain in solution. These are removed periodically so that their concentrations do not build up sufficiently to cause them to also deposit at the cathode. The copper that deposits at the cathode is extremely pure because almost all of the impurities present in the blister copper have been removed
Recount the steps taken to recycle aluminium
The recycling of Aluminium from drink cans, car engines and body trim, boats and appliances is well established in Australia. The Aluminium recovered from these and other sources is sorted according to its alloy type. It is then sent to a smelter, where it is melted in specifically designed furnaces. The molten Aluminium is then analysed and its composition adjusted before being cast into ingots, which are sent to manufacturers who use the recycled Aluminium to make new drink cans, engine parts, garden furniture and other products.

WATER

123. Define the terms solute, solvent and solution
Solutions- mixtures containing quantities of dissolved substances. Solutions are defined as homogeneous mixtures.Solute- the substance that is dissolved in another substance or solutionSolvent- the liquid that does the dissolving
124. Identify the importance of water as a solvent
The vital role of water in the maintenance of life cannot be over- emphasised. Its ability to act as a solvent for many solutes is a particularly important property. Water dissolves, at least to some extent, oxygen, various salts and nutrients, and is therefore important in supporting plant and animal life. Also, many waste products such as carbon dioxide, ammonia and urea are soluble in water, providing an important mechanism by which plant and animal wastes can be removed. The human body is over two – thirds water, with blood, our life- maintaining transport system, being over 80% water. Without water, blood would not be able to flow in arteries, veins and capillaries and transport essential nutrients and waste products. Water is also a major component of our lymph system, and the moisture lining our lungs allows the gases oxygen and carbon dioxide to be transferred between the air we breathe and our respiratory system.
125. Compare the state, percentage and distribution of water in the biosphere, lithosphere, hydrosphere and atmosphere
Liquid water covers nearly 70% of the Earth’s surface in the form of oceans, seas, lakes, ground water and river, which are collectively referred to as the hydrosphere. Water also occurs in vast quantities beneath the surface as ground water stored in aquifers, layers of water- bearing rocks. The Great Artesian Basin from Queensland through NSW and into Victoria and SA is an example.The polar ice caps cover another portion of our planet, dominating the regions north of the Arctic Circle and south of the Antarctic circle. The solid water found in the polar ice caps, above the snowline at higher altitudes and in glaciers, usually contains lower concentrations of dissolved salts because water generally freezes as a pure substance.

Our atmosphere contains varying amounts of gaseous water, water vapour, in the range 0-5%. Although this represents only a small percentage of the atmosphere, it allows for the transport of vast quantities of water through the Earth’s water cycle.
Water in the Lithosphere can occur as moisture or permafrost within solid and rocks, and as water of crystallisation within minerals. Water of crystallisation or water of hydration refers to water molecules that form part of the crystal structure of many ionic substances. Common examples are hydrated copper (11) sulfate, CuSO4.5H2O, which has 5 water molecules associates with each formula unit, and hydrated Sodium Carbonate, Na-2CO3.10H2O. Examples of minerals containing waters of crystallisation include gypsum CaSO4.2H2O, and gibbsite Al2O3.3H2O. the forces holding the water molecules within the crystal structure are not strong, so waters of crystallisation can generally be removed by heating.
Water is the major constituent of living matter, representing approximately 70% of the biosphere. It is a component of all cells, the transport system for nutrient and waste products in living things, a reaction medium for many biochemical processes, and, together with carbon dioxide, is a reactant in photosynthesis.
Biosphere Lithosphere Hydrosphere Atmosphere
Percentage of water
70% Variable 96-100% 0-5%
State of water
Liquid Liquid,Water of crystallisation, solid ice
Liquid, solid ice Gas
126. Outline the significance of the different states of water on Earth in terms of water as:
A constituent of cells and its role as both a solvent and a raw material in metabolism
A habitat in which temperature extremes are less than nearby terrestrial habitats
An agent of weathering of rocks both as liquid and solid A natural resource for humans and other organisms
On Earth water is:127. A necessity for all types of living matter in the form of:
128. A raw material (used in the chemical reactions that constitute life)129. A solvent in which life processes (reactions) occur130. A transport medium for bringing nutrients to cells and carrying waste
products131. A thermal regulator by smoothing out sudden and large temperature

variations A habitat for some life forms such as fish, algae, and bacteria- the place where
they live. Water bodies have the advantage that they show less fluctuation in temperature than do land and air masses.
The major weathering and eroding agent of the Earth. When geological action produces mountains, water erodes them away aver many millennia. There are three processes: Rain and rivers wash loose material to lower altitudes and eventually to sea Glaciers cut a swathe from mountain tops through weaker rocks to the oceans,
and A freeze- thaw mechanism sees liquid water seep into small cracks in rocks,
freeze and expand and so widen the crack until large fragments of rock break away.
The end result is a more gently sloping landscape with o steep mountains. Even in an old geological formation such as Australia, rain and rivers continue to erode the landscape, washing more and more earthly material into the ocean.
A natural resource for humans to use: For drinking, food preparation, washing and recreation For irrigation crops and watering livestock As a working fluid in electricity generating stations and as a coolant in them
and in many industries For generating electricity directly (hydro- electricity) An industry as a reactant, solvent and cleaning agent, and for waste disposal
and settling dust As a mode of transport (ships: which are less important today with railways
and aircraft) For recreational purposes (swimming, boating, skiing) and for aesthetic
enjoyment.
132. Construct Lewis electron dot structures of water, ammonia and hydrogen sulfide to identify the distribution of electrons
133. Compare the molecular structure of water, ammonia and hydrogen sulfide, the differences in their molecular shapes and in their melting and boiling points

H2O (water)
NH3 (ammonia) H2S (hydrogen sulfide)
Melting point (oC) 0 -78 -86Boiling point (oC) 100 -33 -60
134. Describe hydrogen bonding between molecules
HF, H2O and NH3 are all highly polar because they contain three of the most highly electronegative elements, fluorine, oxygen and nitrogen, bonded to hydrogen, which has a relatively low electronegativity. This results in very polar molecules and much stronger intermolecular forces than expected for dipole- dipole interactions. These particularly strong intermolecular forces are referred to as hydrogen bonds.
Hydrogen bonding is a special form of dipole- dipole attraction. It is found in systems where a hydrogen atom is bonded to an atom of Oxygen, nitrogen or fluorine. When hydrogen is bonded to the highly electronegative fluorine, oxygen or nitrogen atoms, the resulting bond is very polar and the shared electrons are strongly attracted towards the more electronegative atom. Because the hydrogen atom in these circumstances has an appreciable partial positive charge, it experiences a strong attractive force with lone pairs of electrons on oxygen, nitrogen or fluorine atoms of nearby molecules. Hydrogen bonding is particularly strong in solid HF, which consists of long chains of HF molecules.Other electronegative elements such as chlorine, bromine and sulphur do not form hydrogen bonds. Although H-Cl, H-Br, and H-s bonds are polar, the attractive force between the partially positive hydrogen atoms and the lone electron pairs on other chlorine, bromine and sulphur atoms is not as strong. The chlorine, bromine and sulphur atoms are much larger and the lone pair electrons are not as accessible to the partial positively charged hydrogen atoms.
The essential requirements for hydrogen bonding are:135. A hydrogen atom bonded to N, O or F do that the hydrogen atom has an
appreciable partial positive charge due to the unequal sharing of the pair of electrons in the covalent bond
136. An unshared pair of electrons on a neighbouring N,O or F atom that can attract the partially positive hydrogen atom.
The strength of hydrogen bonds is in general about 10 times that of dipole- dipole forces but about one- tenth that of ionic or covalent bonds. Hydrogen bonding is important in many chemical systems.
137. Identify the water molecule as a polar molecule

A water molecule is composed of two hydrogen atoms covalently bonded with one oxygen atom. The oxygen atoms attracts the electrons more strongly that the hydrogen atom in each of the covalent bonds. Therefore both O-H bonds are polar. The vector sum of these two dipoles produces a net molecular dipole as shown. Thus the water molecule is a polar molecule. This can be shown to be true by holding a charged rod close to a stream of water running from a tap. The attraction between the rod and the polar water molecules causes the stream of water to deflect.
138. Describe the attractive forces between polar molecules as dipole- dipole forces
If two polar molecules such as HCl approach, they will tend to orient themselves in such a way that the positive end of one molecule is close to the negative end of another molecule. This orientation leads to a lower potential energy.
The force of attraction between the oppositely charged ends of neighbouring polar molecules is called dipole- dipole attraction. The ordered structure is most pronouncd in the solid state. In the liquid state, the molecules are not as ordered, due to their higher kinetic energy.
The charged ends of a dipole have a relatively small charge compared with the charges of iions in ionic substances. Consequently, dipole- dipole attractive forces are relatively weak compared with the attractive forces between oppositely charged ions. Therefore solid HCl, a polar molecular solid, melts at -114oC whereas NaCl, an ionic solid, melts at 801oC.
139. Explain the following properties of water in terms of its intermolecular forces:
Surface tension Viscosity Boiling and melting points
Surface tensionThe water molecules at the surface of a beaker of water are not surrounded by other water molecules in the same way as those molecules in the centre of the beaker. The result is that the molecules on the surface have an overall attractive force downwards into the rest of the water. This downward force creates a tension on the surface of the water, so that it behaves like a tightly stretched skin. This is known as surface tension and is a property of all liquids. The strength of the surface tension is directly proportional to the strength of the forces between the particles of the liquid, so water has a relatively strong surface tension. It is this property that allows many insects to walk on water and a paper clip to float.
Viscosity

Viscosity refer to how easily a fluid (gas or liquid) flows, and is defined as the resistance of a fluid to flow. A thick fluid such as honey is said to have a high viscosity. Blood and motor oil have somewhat lower viscosities. The viscosity of water is lower again, but is higher than that of many other liquids and, of course, air. When poured, viscous fluids flow more slowly than less viscous fluids. Viscous fluids offer more resistance to movement than less viscous fluids. For example, it is more difficult to move through water than air.Viscosity is another property that relates directly to the strength of the forces between the particles of the fluid and to the size of the particles. These forces determine how easily the molecules of the fluid move past each other. The viscosity of water is greater than many other liquids such as kerosene, petrol or acetone because the intermolecular forces are much stronger in water than in other liquids. Viscosity decreases as temperature increases, due to the increased motion of the particles. For this reason motor oil contains additives that change shape on heating. This compensates for the decrease in viscosity of the oil that occurs due to heating.
Melting point and boiling pointThe melting and boiling points of water are much higher than would be anticipated for a molecule of its size. The melting and boiling points of water are much higher than for molecules of similar mass, therefore reflecting the strength of the hydrogen bonds between its small polar molecules. In an ice crystal the covalently bonded molecules are arranged in a regular patter, with each molecule being hydrogen bonded to four other water molecules. When ice melts, heat energy is absorbed, increasing the kinetic energy of the molecules so they can break free of the relatively strong hydrogen bonds in the ice lattice.
140. Explain changes, if any, to particles and account for those changes when the following types of chemicals interact with water:
a soluble ionic compound such as sodium chloride a soluble molecular compound such as sucrose a soluble or partially soluble molecular element or compound such as
iodine, oxygen or hydrogen chloride a covalent network structure substance such as silicon dioxide a substance with large molecules, such as cellulose or polyethylene
When an ionic solid dissolves in water, the crystal structure of the substance breaks down and the ions become distributed throughout the solution. For an ionic solid to dissolve, the following must take place:
141. The electrostatic forces of attraction between the positive and negative ions of the ionic solid must be overcome as the crystal dissolves.
142. Intermolecular forces between some polar water molecules must be overcome, to make space for the positive and negative ions.
143. Attractive forces must form between the positive and negative ions and surrounding water molecules.

An ionic solid will dissolve in water if the strength of the attractive forces between the dissolved ions and water molecules is sufficient to compensate for the energy needed to separate the ions in the solid and the water molecules in the solvent. When ionic substance such as common salt (NaCl), baking soda (NaHCO3), or Epsom salts (MgSO4) dissolve in water, they break up into their basic structural units which are ions. These ions then move freely and independently of one another through the solution.The reason why most ionic compounds dissolve in water is that polar water molecules have a strong attraction for charged ions: the negative end of the water molecule (the O atom) attaches to positive ions- several water molecules per ion- while the positive end (the H atoms) attach to negative ions- again several molecules per ion. We say that the ions become hydrated. SEE DIAGRAM
Polar covalent molecular compounds such as ammonia, glucose, sucrose, ethanol and methanol are very soluble in water. Each of these molecules is able to form hydrogen bonds with the water molecules. The intermolecular forces between these substances and water are similar to those within the separate solute and solvent.
Figure 12.4 (SEE DIAGRAM) shows the formation of hydrogen bonds between the polar –OH group in methanol and H2O molecules. Similar hydrogen bonds are formed between water and the other substance listed above.
While fairly small alcohol molecules like methanol (CH3OH) and ethanol (CH3CH2OH) are very soluble in water, larger alcohols such as 1- hexanol (CH3CH2CH2CH2CH2CH2OH) and 1- decanol (CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2) are mush less soluble. Although these larger molecules still contain a polar –OH group capable of forming hydrogen bonds with water, the size of the non- polar hydrocarbon part of the molecule is much larger. The solubility of the alcohol depends on whether the tendency of the –OH group to dissolve in water is greater than the tendency of the hydrocarbon part of the molecule to remain undissolved. In the smaller alcohol molecules, the influence of the –OH group causes the alcohol to be soluble, but in large alcohol molecules the influence of the hydrocarbon part limits the solubility.
Covalent molecular substances unable to form hydrogen bonds with water molecules will only form dipole- dipole or dispersion forces with water. As a result, these substances are generally less soluble in water and are sometimes described as being partially or slightly soluble. The lesser solubility of these substances occurs because the intermolecular forces between the solute and water molecules are generally much weaker than the hydrogen bonds that form between water molecules in the solvent.
Partially soluble covalent molecules include bother elements and compounds. Elements such as nitrogen and oxygen are non- polar and form only dispersion forces with water. As a result these substances have very limited solubility in water. Covalent molecular compounds may be non- polar or polar. Non- polar compounds such as butane (C4H10) and carbon tetrachloride (CCl4) can only form dispersion forces and therefore have very

low solubility. Polar molecular compounds such as chloroform (CHCl3) and ether (C2H5OC2H5) for dipole- dipole forces with water molecules. These are slightly stronger than dispersion forces, resulting in slightly greater solubility for these substances.
The low solubilities of some gases are due to the weak intermolecular forces, dipole- dipole forces and/ or dispersion forces, between eh gas and water molecules. Some substances such as hydrogen chloride and chlorine are more soluble tan would be expected. This suggests that when they dissolve, these gases undergo some sort of chemical process, thereby increasing their solubility.The covalent molecular gas hydrogen chloride (HCl) is very soluble in water. In this case the HCl molecules undergo ionisation when dissolved in water.
HCL (g) H+ (aq) + Cl-
(aq)
The formation of ions results in a solution of hydrogen chloride being able to conduct electricity. It is therefore classed as an electrolyte.Similarly, chlorine gas reacts with water, to some extent, as represented in the following equation:
Cl2 (aq) + 2H2O (l) HOCl (aq) + Cl- (aq) + H3O+
(aq)
In a similar way, carbon dioxide, hydrogen sulfide and ammonia all react with water to varying extents. Thus the solubility of covalent substance can be significantly influenced by whether they react with water.Both dissociation and ionisation produce ions in solution. The basic difference is that in dissociation of ionic compounds, the ions are already present in the solid and separate to move into solution, whereas in ionisation of a covalent molecular substance, such as HCl, the ions are formed due to the reaction with water.
Covalent network solids such as silicon dioxide and diamond are insoluble in water and most other solvents. This is because the very strong covalent bonds that form the crystal lattices of these substances cannot be broken by the weaker intermolecular forces that could be formed with water molecules.
Large covalent molecules such as cellulose and polyethylene (polyethene) do not dissolve in water or most other solvents. This is because the very strong covalent bonds that form these large molecules cannot be broken by the weaker intermolecular forces (hydrogen bonds, dispersion forces) that could possible be formed with the water molecules. In some cases the molecules have polar components. Despite the presence of these potential hydrogen bond- forming sites, these very large molecules are insoluble due to the presence of the large non- polar portion of the molecule and the need to break vast numbers of hydrogen bonds between the solvent water molecules.
144. analyse the relationship between the solubility of substances in water and the polar nature of the water molecule
A solute will dissolve in a solvent only if the intermolecular forces within the solute and

those within the solvent are similar to those occurring between solute and solvent molecules. Polar solvents tend to dissolve polar solutes and non- polar solvents tend to dissolve non- polar solutes. Water is a polar molecule
Solute
Polar Non- polarPolar Soluble
e.g. sugar will dissolve in waterInsolublee.g. oil will not dissolve in water
Non- polar Insolublee.g. sugar will not dissolve in kerosene
Solublee.g. oil will dissolve in kerosene
145. identify some combinations of solutions which will produce precipitates, using solubility data
Soluble anions ExceptionsNO3
1- None
Cl1- Ag1+ insoluble, Pb2+ slightly soluble
Br1- Ag1+ insoluble, Pb2+ slightly soluble
I1- Ag1+, Pb2+ insoluble
SO42- Ba2+, Pb2+ insoluble, Ca2+, Ag+ slightly soluble
Insoluble anions ExceptionsOH1- Na1+, K1+, Ba2+ soluble; Ca2+ slightly soluble Note: NH4OH
exists as NH3 (aq)
S2- Na1+, K1+, NH41+ soluble
CO32- Na1+, K1+, NH4
1+ solublePO4
3- Na1+, K1+, NH41+ soluble
146. describe a model that traces the movement of ions when solution and precipitation occur
When an ionic substance dissolves in water, it breaks up into ions which move independently through the solution. When a solution becomes saturated, ions still continue to break away from the crystals of solid and do into solution, but in addition an equal number of ion pairs from the solution precipitate out onto the solid. The following experiment demonstrates this.Suppose we have a saturated solution of lead nitrate in contact with lead nitrate crystals. Let us now add some lead nitrate crystals that contain radioactive lead. Radioactive lead is identical with ordinary lead except that it emits a special form of radiation called beta rays which can easily be detected with a radiation counter. We find that just after adding this radioactive lead nitrate solid, all the radioactivity is in the solid: there is none in the solution. However as time passes we find that some of the radioactivity moves into the solution even though the total concentration of lead ions in the solution does not change. Eventually the same fraction of the lead ions in the solution is radioactive as in the solid.This experiment shows that some lead ions move from the solid into the solution, though

this does not change the concentration of lead ions in the solution. This must mean that some lead ions (and nitrate ions) move back from the solution to the solid to balance things out.
147. identify the dynamic nature of ion movement in a saturated dissolution
When a sold is in contact with its saturated solution there is a dynamic balance between dissolution and precipitation: both are occurring, but at equal rates so that there is no overall change in concentration in the solution. We call this dynamic equilibrium. We say that the solid is in equilibrium with the solution. For example when solid lead nitrate is in contact with its saturated solution in water, the lead and nitrate ions in the solid will continue to dissolve at a certain rate and the ions in the solution will continue to precipitate at the same rate so that there is no change in the concentration of ions in the solution.
148. describe the molarity of a solution as the number of moles of solute per litre of solution using:
C = n VThe molarity of a solution is the number of moles of solute per litre of solution
149. Explain why different measurements of concentration are important
A variety of ways of expressing concentration is used because each method has advantages for particular situations. In commerce and industry and in shopping where the main concern is with how much solute is present, when mass per unit volume is very convenient. If we want a desired mass of the solute, we can then just measure out the necessary volume of the solution. It is usually easer to measure out volumes than masses.If the solute is a liquid then volume per unit volume is often preferred because we generally think in terms of volumes of liquids rather than masses.In environmental contexts concentrations are usually quite low. Masses per unit volume or per cent compositions generally lead to very small numbers, so ppm gives more manageable numbers. For example, 1.5 ppm is more convenient to say and write than 0.00015% or 0.0015g per litre (a litre is approximately a kilogram for water).When we want to measure quantities in chemical reaction, we will find that another measure of concentration in terms of moles per litre is more convenient.
150. Explain what is meant by the specific heat capacity of a substance
Specific heat capacity, also called specific heat, is the amount of heat energy required to change the temperature of 1g of a substance by 1 Kelvin. The unit for specific heat

capacity is J g-1 K-1 (or KJ kg-1 K-1).
151. Compare the specific heat capacity of water with a range of other solvents
Water has a high specific heat capacity, which makes it very useful in cooling systems such as car radiators and also home heating systems.
Substance Specific heat
capacity (J K-1 g-1)
Substance Specific heat Capacity (J K-1 g-1)
Water 4.18Ethanol 2.44Ethylene glycol 2.39Glycerol 2.38Acetone 2.17
Ethyl acetate 1.94Hexane 2.26Chloroform 0.96Mercury 0.14Gallium (I, >30oC) 0.37
152. Explain and use the equation
H= -mC T H is the chemical energy released or absorbed (joules) + absorbed, - releasedM is the mass of substance being heated or cooled (g)C is the specific heat capacity of the substance being heated or cooled (J g-1 K-1) T is the temperature change of the substance being heated or cooled (K)
153. Explain how water’s ability to absorb heat is used to measure energy changes in chemical reactions
The heat of reaction is the amount of heat released or absorbed when a chemical reaction occurs. It is generally given in kilojoules per mole of some specified reactant or product (for example as 890 Kj /mol of methane).Heat of reaction is commonly determined by using a measured quantity of water to collect or provide the heat for a known amount of reaction. Measuring the temperature change in the water allows calculation of heat of reaction.The general procedure for calculating the heat of a reaction is
To calculate the molar heat of reaction1) Calculate the amount of heat released or absorbed, generally by using, q= mC T2) Calculate the number of moles that reacted from number of moles = mass molar mass3) calculate heat released or absorbed per mole fromtotal heat releasednumber of moles that reacted

Unfortunately the heat of a reaction depends to some extent on the conditions under which the reaction is carried out, in particular upon whether it is performed at constant volume (in a closed vessel) or at constant pressure (in a container open to the atmosphere). This is particularly true for reactions involving gases. When we compare heats from different reactions, we should do so using constant conditions.
154. Describe dissolutions which release heat as exothermic and give examples
When solutes dissolve in solvents, energy may be absorbed or released. The heat of solution, is the enthalpy change when a solute dissolves in solvent to form a solution. This is usually expressed as the molar heat of solution, and refers to the heat energy absorbed or released when one mole of solute dissolves in solution. Solution processes may be exothermic or endothermic. When ionic substances dissolve in water, there is usually a noticeable change in temperature. When sodium hydroxide dissolves in water, the solution heats up. The dissolution process releases heat which then warms up the solution.Processes which release heat are called exothermicThe dissolution of sodium hydroxide is exothermic. Similarly, dissolving lithium bromide or sulfuric acid in water is exothermic.The dissolution of sodium hydroxide releases 44.5 Kj of heat energy per _mole of NaOH dissolved and is strongly exothermic
NaOH (s) Na+ (aq) + 2OH-
(aq) heat of solution = -44.5 Kj mol-1
155. Describe dissolutions which absorb heat as endothermic and give examples
When potassium nitrate dissolves in water, the solution cools. Dissolving potassium nitrate in water requires an input of energy: this energy is taken from the normal thermal energy of the water and solid substance, sot eh mixture (solution) becomes colder.Processes which absorb heat are called endothermic.The dissolution of potassium nitrate in water is endothermic. Similarly, dissolving ammonium chloride or silver nitrate is endothermic. Ammonium nitrate (NH4NO3) absorbs energy when it dissolves. The equation for its dissolution is represented as follows.
NH4NO3 (s) NH4+
(aq) + NO3- (aq) heat of solution = 25.7 Kj mol-
156. Explain why water’s ability to absorb heat is important to aquatic organisms and to life on Earth generally
Living organisms can survive and reproduce only if their temperatures are maintained within fairly narrow ranges. Water within cells provides the necessary temperature

regulation (that is, it smooths out rapid or large temperature fluctuations). It does this because of its:
157. High heat capacity (much heat absorbed for only a small temperature rise)158. High thermal conductivity relative to other liquids (quickly removes heat),
And because:
159. Water is such a large proportion of most living organisms.
For aquatic organisms the high heat capacity of water means that their environment (oceans, lakes, rivers) maintains a much more stable temperature than the surrounding atmosphere or land. Stable temperatures allow aquatic organisms to thrive.
On a border scale, the fact that water is such a large component of the biosphere means that it (mainly in the form of oceans) has a moderating influence on global temperatures, smoothing out the day-to- night and summer-to-winter fluctuations. This produces a more hospitable environment for all life forms including humans.
160. Explain what is meant by thermal pollution and discuss the implications for life if a body of water is affected by thermal pollution
The moderating effect results from water’s high specific heat capacity. In summer, large quantities of heat energy are required to increase the temperature of water, so water remains relatively cool compared to its surroundings. In winter, large quantities of heat need to be lost to decrease the temperature of water. As a result, water remains relatively warm compared to its surroundings. Water bodies of a reasonable size, such as rivers, lakes and oceans, provide relatively stable temperature conditions for living things. Aquatic organisms generally have not evolved with the temperature control mechanisms, such as perspiration and nocturnal activity, which are characteristic of many land- based organisms.
It is widely known that chemicals can pollute aquatic systems such as rivers and lakes, making them less suitable environments for naturally occurring organisms. It is less well known that excess heat energy discharged into the environment, thermal pollution, can also have a serious effect on aquatic systems and threaten the survival of the aquatic organisms they contain. The heat energy is usually added as a result of an industry that uses water for cooling, such as power generation or steel production. The industrial plant takes in cool water, uses it and then returns hot water to the lake or river. The most important effect of thermal pollution is usually the reduction in the concentrations of dissolved gases such as oxygen and carbon dioxide within the water. In general, the solubility of gases decreases with increasing temperatures.
At the higher temperatures associated with thermal pollution, the concentration of

dissolved oxygen may fall below that necessary to sustain particular life forms that are present. Severe oxygen depletion may be fatal to fish and other aquatic animals. Fish are particularly susceptible to changes in water temperature. At higher temperatures, fish have a higher metabolic rate, requiring a higher concentration of dissolved oxygen, when in fact the levels of dissolved oxygen are lower. Fish such as trout that thrive in cold water will die if the temperature goes up more than a few degrees. Other ecological effects can include:
161. displacement of plankton 162. the food base of aquatic ecosystems, by blue- green algae at higher
temperatures.163. fish eggs do not develop or hatch if temperatures are too high164. false temperature cues are given to aquatic life, thus setting off migration
and spawning at wrong time of the year165. lethal temperature limits may be exceeded (particularly in times of low
flow in rivers166. sudden temperature changes (resulting from turning cooling on or off) can
kill fish eggs even when the temperature change is within the survival range of the eggs.
Thermal pollution is a concern for the electricity generating stations on the shallow lakes along the NSW Central Coast (Lake, Macquarie, Munmorah Lake, and Tuggerah Lake). Thermal pollution is rarely a problem when the open ocean is used because currents and tidal action effectively mix and disperse warm water discharges and so prevent any significant temperature rises.Ways of combating thermal pollution include using cooling towers so that cooling water is completely recycled, and using cooling ponds (artificial lakes used exclusively for the power station).

ENERGY

167. Outline the role of photosynthesis in transforming light energy to chemical energy and recall the raw materials for this process
Using light energy trapped by chlorophyll, plants combine carbon dioxide and water to form carbohydrates and oxygen. Carbohydrates are organic substances containing carbon, hydrogen and oxygen and have the general formula Cx (H2O)y. Carbohydrates can be classified into the following groups:
168. Monosaccharides, e.g. glucose and fructose169. Disaccharides, e.g. sucrose (common sugar) and maltose170. Polysaccharides e.g. starch, glycogen and cellulose
The process of photosynthesis is quite complex and involves many steps but the overall reaction can be represented by the equation:
Carbon dioxide + water light carbohydrate + oxygen
CO2 (g) + H2O light [CH2O] + O2
Because the carbohydrate glucose, C6H12O6, is one of the main photosynthetic products of this reaction, the equation is usually written:
Carbon dioxide + water light glucose + oxygen
6CO2 (g) + H2O (l) light C6H12O6 (aq) + 6O2 (g)
This reaction is endothermic, as it requires the absorption of energy. Light energy from the sun is transformed into chemical potential energy, which is stored within the glucose molecules.The plant will use some of the glucose produced by photosynthesis almost immediately for its own energy requirements, but most is converted into complex carbohydrates (polysaccharides). These include starch, which is used for energy storage, and cellulose, the main component of the cell walls of plants, which provide support and structure.
171. Outline the role of the production of high energy carbohydrates from carbon dioxide as the important step in the stabilisation of the sun’s energy in a form that can be used by animals as well as plants

The chemical energy stored in glucose and other carbohydrates can be released by respiration. This reaction makes this energy available to animals, including humans, when they eat plant material containing these carbohydrates. The chemical breakdown of food releases the chemical potential energy stored within the molecules. Most living things obtain energy by the oxidation of carbohydrates.For example, plants and animals oxidise glucose to produce energy in the process known as aerobic (in the absence of oxygen) respiration.
Glucose + oxygen enzymes carbon dioxide + water + energy
C6H12O6 (aq) + 6O2 (g) enzymes 6CO2 (g) + 6H2O (l) + energy
Humans and other animals can use the energy stored in sugars such as glucose, fructose and sucrose, which are present in plants. We can also breakdown the polysaccharide starch in foods such as potatoes, wheat, corn and rice, to form glucose. Ruminants, such as cows and sheep, can also break down cellulose into its constituent glucose units. This is not possible for humans, for whom cellulose is indigestible but an important source of dietary fibre.
172. Identify the photosynthetic origins of the chemical energy in coal, petroleum and natural gas
CoalCoal is by far the most abundant fossil fuel. Most of the Earth’s coal deposits were formed between 350 and 225 million years ago during the Carboniferous and Permian periods. Coal is produced by the accumulation of plant material in conditions where decomposition by oxidation is negligible. The stagnant waters often associated with swamps, marshes and mangroves are an ideal environment for this process. During the Carboniferous and Permian periods there was an abundance of plant life. Plant debris including leaves, bark and wood accumulated in these ancient swamps and was buried beneath more plant material.Initially the organic matter was converted into peat, a soft, spongy material containing large amounts of water. With increased pressure and temperature, peat was converted into brown coal (lignite) then black coal (bituminous coal) and finally anthracite. The first stage in the formation of coal is biochemical decomposition caused by anaerobic bacteria. This results in the loss of volatile compounds such as CO2, H2O and CH4, with an overall increase in the relative carbon content. The second phase, coalification, occurs when the peat is buried and compressed by the decomposition of sediments. In coalification, the combined effects of temperature and pressure over long periods of time reduce the oxygen content (through release of CO2) and hydrogen content (through release of CH4). This results in increasing carbon content through the sequence: peat lignite bituminous coal anthracite. The type of coal produced depends on the degree to which the original material has been changed. For example, low- grade peat can contain carbon, hydrogen and oxygen atoms in the ratios 1:2:1, while in high- grade anthracite the carbon: hydrogen ratio can be as high as 5:2, with virtually

no oxygen present.Coal contains numerous impurities in varying levels, particularly sulphur and sediments such as mud, deposited and buried with plant material. When the coal is burnt, these impurities form pollutants including sulphur dioxide and ash. The sulphur content of Australian coals is fairly low and our coal is therefore in great demand.
PetroleumPetroleum consists of a complex mixture of hydrocarbons. Before refining, petroleum is a viscous black liquid often called crude oil. Its composition varies from well to well. Like coal, the origins of the chemical energy in petroleum can be traced back to light energy from the sun. This is because most petroleum is derived from the buried remains of marine organisms, particularly bacteria and plankton. The oils and fats in the bodies of these organisms are converted into hydrocarbons by high temperatures and pressures deep in the Earth’s crust. Due to its density, the oil tends to migrate upwards, where it may seep out onto the Earth’s surface or become trapped beneath impervious rock. In the latter case accumulation of oil and gas occurs, forming an oil field.
Natural gasNatural gas, like petroleum, has its origin in marine organisms that accumulated as organic sediment in oceans or inland seas. Over long periods of time this organic matter was covered by further sediment. The action of anaerobic bacteria and the increasing temperature and pressure of compaction at depth reduced the organic material to hydrocarbons. These hydrocarbons accumulated in porous pockets in the upper strata of the Earth, trapped by layers of impervious rock. Natural gas is often found in conjunction with petroleum but can be found by itself, as in the vast reserves currently being developed on the North- west Shelf in Western Australia. Part of the gas produced by these off- shore wells is piped to Perth and other parts of the state, where it is burnt for direct heating or as a fuel in electricity generation. Vast quantities are also liquefied and exported overseas as liquefied natural gas (LNG).Natural gas contains a range of compounds, particularly alkanes of low molecular mass. Methane (CH4) is the main component of natural gas, although ethane (CH3CH3), propane (CH3CH2CH3), butane (CH3CH2CH2CH3), carbon dioxide, nitrogen and hydrogen sulfide are also present in varying amounts, depending on the source of the gas. Generally ethane, propane and butane are extracted from natural gas. The ethane is used in the petrochemical industry, while propane and butane are liquefied under pressure to produce liquefied petroleum gas (LPG).Natural gas is a relatively cheap, clean and convenient fuel. The burning of natural gas can generally be represented by the burning of methane.
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g)
173. Identify the position of carbon in the periodic Table and describe its electron configuration
Carbon has an atomic number of 6 and is found in group 4 of the periodic table. The most

common isotope of carbon has a mass number of 12. Hence the nucleus of a carbon- 12 atom contains 6 protons and 6 neutrons. 6 electrons are distributed in the space around the nucleus of each carbon atom. In the ground or lowest- energy state, a carbon atom has an electron configuration of 2,4.Carbon has 4 valence electrons and tends to form 4 bonds to achieve a share in a valence electron octet. For example in methane, CH4, carbo forms 4 single covalent bonds with 4 separate hydrogen atoms. In carbon dioxide, CO2, the carbon atom forms 2 double covalent bonds with 2 oxygen atoms.
174. Describe the structure of the diamond and graphite allotropes and account for their physical properties in terms of bonding
graphiteDiamond, a crystalline form of pure carbon, is an example of a covalent network substance. It is widely used in jewellery because when cut and polished it sparkles brilliantly. Experimental evidence indicates that in diamond all the C-C bonds are of the same length and all the bond angles are 109.5o. Using this information a diamond crystal can be pictured as a single giant molecule made up of a regular network of carbon atoms extending throughout the crystal. Each carbon atom can be imagined to be at the centre of a regular tetrahedron, surrounded by 4 other carbon atoms at the corners of the tetrahedron.In this 3- dimensional network of atoms, each carbon atom forms 4 covalent bonds by sharing electrons with each of its 4 nearest neighbours. The bonding electrons are tightly bound and highly localised and are therefore unable to move freely within the lattice. Therefore diamond crystals are non- conductors of electricity. It is also very difficult to distort diamond, and other covalent network crystals, since this would involve breaking many strong covalent bonds. Consequently, diamond is extremely hard and has a very high melting and boiling point. If the highly directional bonds in diamond are subjected to extreme stress, the crystal is unable to deform in shape and it shatters. Diamond is the hardest known naturally occurring substance. Diamonds unsuitable for gemstones are used in applications such as glass cutting and polishing, mineral exploration drills and dentists’ drills.
GraphiteGraphite, a second form or allotrope of pure carbon, has properties quite different from those of diamond. In fact, the properties of graphite are quite unusual for a covalent network substance. Graphite has a high melting and boiling point but is soft and is a good conductor of electricity.In graphite, the carbon atoms are arranged in flat parallel layers. Within each layer each carbon atom is covalently bonded to 3 other atoms, forming a hexagonal arrangement. Each layer can be considered a 2- dimensional network of carbon atoms. The strong covalent bonds within the layers account for the very high melting point of graphite.Each carbon atom shares one of tis valence electrons with each of its 3 neighbouring atoms within the plane. The 4th electron is delocalised. The delocalised electrons are free to move parallel to the layers of carbon atoms but not perpendicular to the layers. The

delocalised electrons are responsible for the electrical conductivity of graphite. Because of its electrical conductivity, graphite is used as an electrode in dry cells, in electric furnaces and in electrolytic cells.The bonding between layers in graphite is relatively weak, consisting only of dispersion forces. As a result, the layers can easily slide over each other. This accounts for the softness and slippery feel of graphite and its use as a dry lubricant. Graphite mixed with clay is also used to make the ‘lead’ in lead pencils. When a lead pencil is used, the graphite is transferred to the paper. Graphite fibres are used extensively to improve the strength of polymers and other plastics. These composite materials are used in sporting equipment such as golf clubs, tennis racquets and fishing rods, and in advanced aircraft.
175. Identify that carbon can form single, double or triple covalent bonds with other carbon atoms
The 2 simplest molecules containing carbon- carbon single bonds are ethane (CH3CH3) and propane (CH3CH2CH3). In these compounds each carbon atom forms 4 single bonds, which again have a tetrahedral orientation. In the case of CH3CH3 3 of the bonds formed by the carbon atoms are C-H bonds, while the other bond is a C-C bond.
Carbon- Carbon double bondsThe compound ethene or ethylene (CH2CH2) is the simplest carbon compound containing a C=C double bond. In this case only 2 of each carbon atom’s 4 valence electrons are used in bonding with hydrogen atoms. Hence each carbon atom shares 2 pairs of electrons with another carbon atom. These 2 pairs of electrons constitute a double bond.An examination of compounds such as ethene (CH2CH2) indicates that the bond angles are 120o, and the geometric arrangement of the 2 carbon atoms and adjoining hydrogen atoms in planar. This again can be explained in terms of the VSEPR theory. In using the VSEPR theory, the C=C double bond is viewed as a single region of charge. To minimise electron repulsion, the 3 electron regions around each carbon atom adopt a planar orientation with bond angles of 120o.
Carbon- carbon triple bondsEthyne (CHCH) is the simplest carbon compound containing a C C triple bond. Each carbon uses only one electron in forming a bond with a hydrogen atom. This allows the carbon atoms to share 3 pairs of electrons, resulting in the formation of a triple bond. An examination of compounds such as ethyne (CHCH) indicates that the bond angles are 180o. If the C C triple bond is taken to represent one region of charge, then by applying the VSEPR theory the shape of such molecules must be linear.
As the number of bonds between the 2 carbon atoms increases, the bond length decreases. This occurs because of the increase attraction between the bonding electrons and the carbon nuclei. The bond angles and the geometric arrangement of atoms can be determined by using the VSEPR theory.
Type of carbon- Representation Number of sharedPairs of electrons
Bond angle

carbon bond
Single -C-C- 1 109.5o
Doube -C=C- 2 120o
Triple -C C- 3 180o
176. Explain the relationship between carbon’s combining power and ability to form a variety of bonds and the existence of a large number of carbon compounds
In organic compounds, carbon atoms almost always form 4 bonds. This suggests that the carbon atom’s 4 valence electrons are all involved in bonding. An examination of simple carbon- based molecules like methane (CH4) and carbon tetrachloride (CCl4) indicates that in these compounds the carbon atoms forms 4 identical single covalent bonds and that the angles between the bonds are 109.5o. It can be predicted from the valence shell electron pair repulsion (VSEPR) theory that a tetrahedral orientation of the electron pairs is required, to minimise the electrostatic repulsion between them.The central role of carbon in organic chemistry depends on the fact that carbon has a valency of 4 and can form a variety of bonds. Consequently, carbon atoms can form chains of virtually unlimited length containing many carbon- carbon bonds. The valence electrons not involved in forming carbon- carbon bonds are used in forming bonds with atoms of other elements such as hydrogen, oxygen, nitrogen and halogens.
177. Describe the use of fractional distillation to separate the components of petroleum and identify the uses of each fraction obtained
Petroleum consists of a complex mixture of compounds that are mainly hydrocarbons in the C1 to C40 range. While alkanes are the principal component, petroleum also contains smaller amounts of alkenes, aromatic compounds (carbon compounds containing a benzene ring, C6H6) and other organic compounds containing oxygen, nitrogen and sulfur. The actual composition of crude oil varies depending on its source.The petroleum must be separated into several fractions to make maximum use of the variety of constituents it contains. This is achieved by the process of fractional distillation using a fractionating tower. This separates the petroleum into fractions containing hydrocarbons with similar boiling points.The petroleum is heated to about 400oC to produce a hot liquid/ vapour mixture that enters the fractionating tower. Inside the tower are horizontal trays, each of which contains many bubble caps. As the vapour rises it forces up the bubble caps and bubbles through the condensed liquid in the trays. The higher boiling point components tend to condense to liquid while the lower boiling point components continue to rise up the tower. The lower the boiling point of a component, the higher its vapour will rise in the fractionating tower before it condenses. Because the intermolecular forces involved are only weak dispersion forces, the boiling point depends mainly on the molecular size and shape. The lighter hydrocarbons will condense towards the top of the tower while heavier ones will condense nearer the bottom.The fractionating tower is designed so that the various fractions can be drawn off as they

condense. (SEE PICTURE IN TEXT BOOK)
Fraction Principal uses and comments
Refinery gas Used mainly as a fuel or as starting material in the manufacture of plastics
and petrol additives
Gasoline
(naphtha,
petrol)
Motor fuel, naphtha for manufacture of petrochemicals, solvents
Kerosene Aviation fuel, starting material for catalytic cracking process to produce
other organic compounds
Diesel and gas
oils
Diesel fuel, starting material for catalytic cracking process to produce
other organic compounds
Lubricating
oils
Lubricating oil, starting material for catalytic cracking process to produce
other organic compounds
Paraffin
waxes
Candles, wax paper
Bitumen Roofing tar, road bitumen (asphalt)
178. Identify and use the IUPAC nomenclature for describing straight- chained alkanes and alkenes from C1 to C8
ALKANESNumber ofCarbon atomsIn chain
MolecularFormula
Structural formula Name
1 CH4 CH4 Methane2 C2H6 CH3CH3 Ethane
3 C3H8 CH3CH2CH3 Propane4 C4C10 CH3(CH2)2CH3 Butane5 C5H12 CH3(CH2)3CH3 Pentane6 C6H14 CH3(CH2)4CH3 Hexane7 C7H16 CH3(CH2)5CH3 Heptane8 C8H18 CH3(CH2)6CH3 Octane9 C9H20 CH3(CH2)7CH3 Nonane
ALKENESNumber ofCarbon atoms
MolecularFormula
Structural formula Name

In chain2 C2H4 CH2=CH2 Ethene3 C3H6 CH3-CH=CH2 Propene
CH3-CH2-CH=CH2 1- butene
CH3-CH=CH-CH3 2- buteneCH3-CH2-CH2-CH=CH2 1- penteneCH3-CH2-CH=CH-CH3 2- pentene
6 C6H12 CH3CH2CH2CH2CH=CH2 1- hexeneCH3CH2CH2CH=CHCH3 2- hexeneCH3CH2CH=CHCH2CH3 3- hexeneCH3CH2CH2CH2CH2CH=CH2 1- heptaneCH3CH2CH2CH2CH=CHCH3 2- hepteneCH3CH2CH2CH=CHCH2-CH3 3- heptaneCH3CH2CH2CH2CH2CH2CH=CH2 1- octaneCH3CH2CH2CH2CH2CH=CHCH3 2- octaneCH3CH2CH2CH2CH=CHCH2CH3 3- octaneCH3CH2CH2CH=CHCH2CH2CH3 4- octane
179. Compare and contrast the properties of alkanes and alkenes C1 to C8 and use the term ‘homologous series” to describe a series with the same functional group
Homologous seriesAlkanes: CnH2n+2
Alkenes: CnH2n
Name RelativeMolecular
Mass
Number ofelectrons
MeltingPoint (oC)
BoilingPoint (oC)
State under normalAtmosphereic
conditions
Methane 16.0 10 -182 -162 Gas
Ethane 30.1 18 -183 -89 Gas
Propane 44.1 26 -188 -42 Gas
Butane 58.1 34 -138 0 Gas
Pentane 72.1 42 -130 36 Liquid
Hexane 86.2 50 -95 69 Liquid
Heptane 100.2 58 -91 98 Liquid
Octane 114.2 66 -57 126 Liquid

From the table it can be seen that there are trends in the melting and boiling points of the alkanes that are related to the size of the molecules. The melting and boiling points increase with increasing relative molecular mass. At 25oC and one atmosphere pressure, the first 4 alkanes are gases, the C5 to C17 are liquids, and alkanes with 18 or more carbon atoms are solids.Alkanes are non- conductors of electricityThe alkenes and alkynes exhibit similar trends in melting and boiling points. This is evident from the fact that at room temperature the smaller members (C2to C4) are gases, the intermediate members (C5 to C15) are colourless liquids and the larger members (>C16) are waxy solids.
These trends in melting and boiling points can be explained in terms of the intermolecular forces between hydrocarbon molecules. Carbon and hydrogen have similar electronegativities and the arrangement of hydrogen atoms around carbon atoms is fairly symmetrical. As a result, hydrocarbon molecules have very low polarities and are usually considered to be non- polar. The intermolecular forces present in hydrocarbons are therefore weak dispersion forces. These dispersion forces increase in strength with increasing relative molecular mass.
The shapes of molecules also influence the strength of dispersion forces. This can be seen by comparing the boiling points of pentane (36.1oC) and dimethylpropane (9.5oC). As these 2 compounds have the same molecular formula and molecular mass, the difference in boiling points must be due to the difference in shape of the 2 molecules. The strength of the dispersion forces is greater for pentane than dimethylpropane. This is because there is relatively greater surface contact between pentane molecules, which have a chain- like structure, than dimethylpropan, whose branched- chain structures results in a more spherical shape.
The densities of hydrocarbons increase gradually with increasing relative molecular mass. For example, the density of pentane (C5H12) is 0.621g cm-3, decane (C10H22) is 0.726g cm-3. Similar trends exist for alkenes and alkynes. All these hydrocarbons have densities less than that of water.
Solutes tend to dissolve in solvents if the solute- solvent intermolecular forces are similar to those within the solute and those within the solvent. In solvents such as water, the intermolecular hydrogen bonds between water molecules are much stronger than the dispersion forces that would form if a non- polar solute dissolved in it. Hydrocarbons are therefore virtually insoluble in water. This, together with their relative densities, is the reason that crude oil is often found floating on top of water in geological formations and why oil spills can have catastrophic effects on bird and aquatic life.
The solubility of hydrocarbons in non- polar solvent is the reason that white spirits (a mixture of alkanes) is a commonly used solvent in paints and varnishes, and non- polar solvents are widely used in dry cleaning to remove grease and oil stains.
The volatility of s substance is the ease with which it can be converted to a vapour.

Volatility increases as boiling point decreases. So for alkanes, volatility decreases as molecular weight increases.The properties of alkenes are similar to those of alkanes. These properties arise because alkenes contain only C-C and C=C bonds which are non- polar and C-H bonds which are just slightly polar. This means that alkene molecules are non- polar so that the only intermolecular forces are weak dispersion forces. This explains their low boiling points and their insolubility in water.
180. Explain the relationship between the melting point, boiling point and volatility of the above hydrocarbon, and their non- polar nature and intermolecular forces (dispersion forces)
181. Assess the safety issues associated with the storage of alkanes C1 to C8 in view of their weak intermolecular forces (dispersion forces)
The main danger associated with the use and storage of hydrocarbon fuels is that they are highly flammable. Consequently there is always a risk of explosion. Explosive reactions, which occur as a result of rapid combustion, produce large quantities of heat energy, causing nearby gases to expand rapidly. Explosions are most likely to occur with fuels that vaporise readily, producing a fuel vapour: air ratio favourable for explosive combustion, and that are easily ignited. Therefore the most important indicators of the potential dangers of a fuel are its flash point and volatility. All the C1 to C8 hydrocarbons are highly flammable and must be used, stored and transported in such a way that vapours cannot build up to form an explosive fuel vapour/ air mixture.
Although natural gas is often piped from gas fields to industry and metropolitan users, and ethyne (acetylene) is usually stored and transported under pressure in gas cylinders, most hydrocarbon fuels are stored and transported as liquids. This is an economical method of transportation because the hydrocarbons occupy much smaller volumes in the liquid than the gaseous state. For example, liquefied natural gas (LNG), methane, is transported overseas from the North- West Shelf in massive ocean tankers. Liquefied petroleum gas (LPG), mainly propane and butane, is also stored and transported under pressure in strong steel cylinders to keep it liquefied at normal temperatures. Pentane, hexane, heptane and octane are volatile, flammable liquid hydrocarbons. They must be stored and transported in sturdy metal or high- density plastic containers that have a narrow neck to reduce evaporation, and tight- fitting lids.
All these fuels have very low flash points and must be stored in cool, well- ventilated areas and kept away from naked flames and sparks, which would ignite them. The valves, regulators and the cylinders themselves must be checked and tested regularly. A foul- smelling compound is added to natural gas so that leaks can be detected before explosive mixtures are produced.
Solid fuels such as coal, coke and wood have low volatilities and are generally less easily

combustible. However, coal dust/air mixtures can have low flash points due to the large surface area of the dust.
Some of the safety measures employed in the storage and transportation of hydrocarbons include:
182. Well maintained cylinders and fittings for gaseous hydrocarbons Methane and ethane are non- condensable gases at room temperature and are therefore stored and transported in high- pressure cylinders which need regular testing and checking. They must be used with proper valves and regulators and stored in well- ventilated places.Propane and butane are condensable with moderate pressures at ambient temperatures. Cylinders for their storage and transport need not be so sturdy but still need to be handled with care, and checked regularly. Because of the flammability of the gases, the cylinders should be stored outdoors or on the outside of caravans and campervans.
183. Added odours for early detectionBecause these gases are so flammable and form explosive mixtures with air, the commercial products, natural gas and LPG, are dosed with a nasty- smelling substance so that leaks can be detected before explosive mixtures develop
184. Sturdy containers for liquidsLiquid hydrocarbon mixtures such as petroleum either, petrol and kerosene are quite volatile and highly flammable. Hence they need to be stored in sturdy containers (metal rather than plastic) with narrow mouths (to restrict evaporation)
185. Minimise the quantities in everyday useOnly the minimum quantities for immediate needs should be stored in laboratories, homes or other enclosed places. Bulk quantities of such liquids should be stored out of the sun (to avoid overheating) but in places which are well ventilated.
186. Do not handle these liquids in confined spacesExplosive mixtures with air can easily build up if these liquids are poured from one container to others in confined spaces. They should be handled outdoors whenever possible. As an example, boats with outboard motors are often safer than ones with inboard motors.
187. Keep hydrocarbons away from naked flamesWhen handling hydrocarbons it is absolutely essential to exclude all naked flames, cigarettes and hot filaments, and to switch off equipment capable of generating sparks (such as electric motors).
188. Use fume hoods for prolonged useWhen using hydrocarbons as solvents in industry on a regular basis, proper fume hoods must be used to minimise inhalation of these toxic substances by the operators. To prevent absorption through the skin, protective gloves should be used
189. At home keep areas well ventilated and avoid inhalationWhen consumers use products containing these compounds (such as rubber cements, other household adhesives, insecticides, cleaning solvents and so on)

they should avoid inhaling the vapours by providing as much ventilation as possible. They should avoid body contact and try to avoid using them in confined spaces.
For motor cars and transporting fuels190. The fuel tank of motor cars is located at the end remote from the hot
engine and is outside the main shell of the car.191. It has narrow inlet and outlet pipes, which are both at the top of the tank to
minimise chances of leakage during accidents; fuel has to be pumped from the tank by the engine, so that in most cars even a fuel line rupture will not cause rapid leakage of petrol.
192. When petrol is transported by road or rail, heavy steel tanks are used which can be well sealed and which are designed to withstand most collisions or overturnings without rupture of the inlet or outlet valves, and therefore without spillage.
193. Features are built into petrol tankers to dissipate any static electricity which could cause sparks during filling and emptying.
194. As a further safety precaution, some commonly used fuels are coloured with added dyes for easy identification (pure hydrocarbons are colourless0. Petrol is coloured yellow (unleaded) or pink (leaded), and kerosene is blue.
All these safety precautions are necessary because hydrocarbons are so volatile. This is because there are only weak dispersion forces between molecules of these non- polar substances.
195. Transportation of gases in strongly constructed pipelines or pressurisation to condense them into liquid form
196. Sturdy construction and chaining of gas cylinders197. Prohibition of flames or sparks of any sort near petrol stations or fuel
storage areas198. Fitting of road and rail tankers with earthing systems to prevent the build-
up of static electricity199. Storage of minimal quantities of fuels200. Regular checking of tanks and pipelines for leaks201. Addition of odorous components in natural gas so that leaks are evident
202. Describe the indicators of chemical reactions
Chemical changes are those in which new substances with different compositions and properties are formed. The combustion of petrol in car engines and methane in gas ovens and stoves are examples of chemical changes. In both cases the new substances carbon dioxide and water are produced. Evidence that a chemical reaction has taken place includes the following:
203. A permanent change in colour204. A change in odour

205. Production of gas bubbles206. The formation of a precipitate207. Release or absorption of energy
208. Identify combustion as an exothermic chemical reaction
The combustion reactions of hydrocarbons are strongly exothermic, indicating that the enthalpy of the products, usually carbon dioxide and water, is much less than that of the reactants. In the combustion of methane, for example,
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) + 890 Kj
There is a decrease in enthalpy of the system of 890 Kj for every mole of methane that burns. For every methane molecule that burns, four C-H and two O=O bonds are broken, and two C=O and four O=H bonds are formed. The large decrease in enthalpy indicates that much less energy is needed to break the bonds in the reactants than is released when the product molecules are formed.The molar heat of combustion of a substance is the heat released when one mole of a substance undergoes complete combustion with oxygen at a constant pressure of one atmosphere (or 100 kPa). For hydrocarbons, it is the heat released when one mole of the hydrocarbon is burned in oxygen to produce carbon dioxide and water. All combustion reactions release energy and are therefore exothermic.
209. Outline the changes in molecules during chemical reactions in terms of bond- breaking and bond- making
All chemical reactions involve the breaking and making of chemical bonds. If the energy required to break bonds in the reactants is greater than the energy released when new bonds are formed to make products, then the reaction will be endothermic. If, on the other hand, the energy absorbed to break bonds is less than the energy released when bonds formed, the reaction will be exothermic.Consider the reaction between hydrogen and oxygen.
2H2 (g) + O2 (g) 2H2O (g) + energy
this reaction can be considered to involve the breaking of two H-H bonds and one O=O bond and the formation of four O-H bonds. The overall reaction involves the release of heat energy, so the energy released from forming the four O-H bonds must be greater than that needed to break the H-H and O=O bonds. The product molecules therefore have a lower enthalpy than the reactants.
210. Explain that energy is required to break bonds and energy is released when bonds are formed

The energy effects associated chemical reactions can be considered to result from the breaking of some bonds and the formatio of others. For example, the breaking of the covalent bond in a HCl molecule is a process that absorbs energy. Conversely, the formation of a H- Cl bond releases energy. From the law of conservation of energy, the quantity of energy released when a H- Cl bond forms is the same as that absorbed when such a bond is broken. The energy absorbed when a chemical bond is broken leads to an increase in the enthalpy of the system. The individual atoms that result represent a higher enthalpy state than when they were combined in the molecule. Alternatively, if the same bond is re- formed, the same amount of energy is released as the system changes back to a lower enthalpy state.
211. Describe the energy needed to begin a chemical reaction as activation energy
For a reaction to occur between reactant molecules, they must collide with a certain minimum energy. Unless this minimum collision energy is exceeded, the colliding molecules will simply rebound and move away form each other. The minimum energy that is required for a collision to result in a reaction is known as the activation energy for the particular reaction. Some reactions have relatively low activation energies and so react at a significant rate at room temperature. For these reactions, a noticeable reaction occurs as soon as the reactants are mixed. For example, a piece of sodium metal is added to water at room temperature produces a violent reaction almost instantaneously. Other reactions occur to an almost insignificant extent at room temperature. In such reactions the activation energy is so high that it is very unlikely that reactant molecules will collide with sufficient energy to undergo reaction. For example, methane will not react with oxygen unless the mixture is ignited. There is a relatively high activation energy for the reaction between methane and oxygen. Despite the fact that under standard conditions there would be in the order of 1010 collisions per second between reactant molecules, virtually none of these would have sufficient energy for a reaction to take place.
212. Describe the energy profile diagram for both endothermic and exothermic reactions
An energy profile diagram is a way of representing the enthalpy (energy) changes that occur during a chemical reaction. The energy difference between the reactants and products represents the heat of reaction. Between the reactant and product states is a very high energy state known as the transition state or activated complex. The transition state represents the highest energy state for the reacting system and corresponds to some stage in the reaction at which bond- breaking and bond- formation are talking place. The transition state is an unstable state and cannot have any more than a very temporary existence. Once formed it will quickly convert to products or return to reactants. The activation energy for the reaction is the difference in the enthalpies of the reactants and the transition state. SEE DIAGRAMSEndothermic- graph shows gain in enthalpyExothermic- graph shows loss in enthalpy

213. Explain the relationship between ignition temperatures and activation energy
All reactions, even exothermic reactions, require an initial input of energy to start the react. For example, the combustion of methane requires an ignition temperature of 630o, or a flame or spark to initiate the reaction. The minimum energy required by reacting molecules before they will react is known as the activation energy (EA). The activation energy overcomes an energy barrier for the reaction and varies from one reaction to another. If the activation energy for a combustion reaction is relatively low, the ignition temperature will also be low. Combustion reactions with higher activation energies will have higher ignition temperatures. The activation energy of both endothermic and exothermic reactions can be shown on an energy profile diagram. The minimum temperature at which a fuel/air mixture spontaneously ignites is called the ignition temperature of the fuel. (SEE GRAPHS ALSO ENTHALPY GRAPHS FOR EXO AND ENDO)
214. Identify the sources of pollution which accompany the combustion of organic compounds and explain how these can be avoided
Carbon monoxide and sootIf insufficient air is available for the complete combustion of a fuel (to carbon dioxide and water), then some carbon monoxide and/or soot is formed. For example when pentane is burnt in a plentiful supply of air, the reaction is:
C5H12 (g) + 8O2 (g) 5CO2 (g) + 6H2O (g)
If insufficient air (oxygen) is available, one possible reaction is:
C5H12 (g) + 6O2 (g) CO2 (g) + 6H2O (g)
Depending on conditions the number of molecules of CO formed per molecule of pentane could range from zero to 5. If even less oxygen was available, the reaction could be:
C5H12 (g) + 4O2 (g) 3C (s) + 2CO (g) + 6H2O (g)
Formation of carbon monoxide is common in petrol engines where the air- to- fuel ratio is close to the stoichiometric value. Diesel engines and electricity generating stations use a much higher air to fuel ratio and so produce very little carbon monoxide. Badly maintained diesel engines can produce a lot of soot under certain conditions.The way to minimise production of carbon monoxide and soot is to keep the air- to- fuel ratio high. This is not possible in petrol engines (ignition becomes too difficult) so emission of carbon monoxide from cars is minimised by using a catalyst in the exhaust to convert any CO formed to CO2.

Sulfur Dioxide
Sulfur dioxide is produced from impurities in the fuel- most commonly from coal. Coal normally contains from 0.5 to 5% sulphur, though Australian coals generally have lower amounts- 0.5 to 2%. When coal burns this sulphur is converted to sulphur dioxide, a pungent gas which cause breathing difficulties at quite low concentrations:
S (s) + O2 (g) SO2 (g)
Removing sulphur from coal is difficult, so power stations try to use low- sulphur coals whenever possible (which is why there is high demand for Australian coals). Alternatively sulphur dioxide has to be removed from the effluent gas at power stations: this is expensive.When crude oil and natural gas are processed for use, virtually all of the sulphur- containing compounds are removed.
Oxides of Nitrogen
Nitrogen and oxygen gas do not normally react with each other. However at high temperatures (above about 1000oC) they combine to for nitric oxide:
N2 (g) + O2 (g) 2NO (g)
Nitric oxide reacts slowly with oxygen to form nitrogen dioxide:
2NO (g) + O2 (g) 2NO2 (g)
These reactions occur in petrol and diesel engines and in power stations. Consequently motor vehicles and power stations emit small concentrations of both of these gases. The term oxides of nitrogen is commonly used to describe this mixture. Sometimes the abbreviation NOx or NOx is used for this mixture.Nitrogen dioxide causes respiratory difficulties and damages organ tissue. However the main concern with nitrogen dioxide is that under the influence of sunlight it leads to the production of ozone, a far more dangerous substance, in what is called photochemical smog.Today most new cars use a catalyst to remove nitric oxide by speeding up the reaction:
2NO (g) + 2CO (g) N2 (g) + 2CO2 (g)
Diesel engines produce more oxides of nitrogen than petrol engines, though currently (2000) there have been no restrictions on exhaust emissions from diesel vehicles.Until recently emissions of oxides of nitrogen from power stations have been tolerated by locating power stations away from population centres. However there is a growing trend towards using catalysts to remove oxides of nitrogen.
Photochemical smog Photochemical smog is most likely to occur on warm, sunny days when the air is very

still. It occurs through a complicated series of reactions involving unburned hydrocarbons and nitrogen oxides. Motor vehicles are mainly responsible for the formation of photochemical smog. Such smog occurs as a brown haze and causes reduced visibility, eye and bronchial irritation, damage to plants and animals, and deterioration of materials.Unburned hydrocarbons in petrol are released into the atmosphere as part of motor vehicle emissions. Nitrogen oxides are formed in car engines because the high temperatures cause nitrogen and oxygen to react. The reaction can be represented as above. It is the brown nitrogen dioxide that gives photochemical smog its characteristic brown colour.The release of hydrocarbons and initial formation of NO and NO2 is followed later in the day by increasing levels of ozone and other pollutants. The nitrogen dioxide absorbs ultraviolet radiation from the sun, to nitric oxide and oxygen atoms. The oxygen atoms combine with molecular oxygen to form ozone.
NO2 (g) + ultraviolet radiation NO (g) + O (g)
O2 (g) + O (g) O3 (g)
Additional reactions between unburned hydrocarbons and ozone or oxygen atoms produce a range of organic compounds. These include aldehydes such as ethanal, CH3CHO, and peroxyacetyl nitrate or PAN, CH3CO-OO-NO2.The increasing occurrence of photochemical smog in Australian cities is quite apparent. This is mainly the result of the high level of car use by people living in these cities. Reducing photochemical smog will depend on improving technology and changing public attitudes towards the use of motor vehicles. Improved engine design and the use of catalytic converters are reducing the emissions of unburned hydrocarbons and nitrogen oxides. In large cities, however, it will also be necessary to reduce the use of petrol- fuelled moto vehicles as the main method of transport.
ParticulatesParticulates are very small particles of solids and/or droplets of liquid. The particles or droplets are so small that they do not easily settle out of the air. They are the most obvious form of air pollution because they affect visibility. They frequently contribute to respiratory problems for aged and frail people ad for asthma sufferers.Although vehicles produce some particulates, the major sources are from industry and power generation, in particular from burning coal and the higher boiling point fractions of crude oil (gas oil or heating oil). From coal and oil, particulates arise from the incomplete combustion of the fuel (as soot did). From coal they also form from the incombustible inorganic matter present in coal to a small extent.Emissions of particulates from stationary sources (power stations and industries) is generally minimised by using electrostatic precipitators. These are devices which use a high voltage to cause small particles to combine with big ones which can be filtered out of the exhaust gas.
215. Describe chemical reactions by using full balanced chemical equations

to summarise examples of complete and incomplete combustion
Complete combustionWhen ignited with a plentiful supply of oxygen, hydrocarbons undergo complete combustion to produce water, carbon dioxide and a large quantity of heat. This makes hydrocarbons very useful as fuels.The following equations represent the complete combustion of methane (natural gas) and butane
Methane + oxygen carbon dioxide + water + energyCH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g) + energyButane + oxygen carbon dioxide + water + energyC4H10 (g) + 13O2 (g) 4CO2 (g) + 5H2O (g) + energy
2
Incomplete combustionThe equation for the combustion of butane indicates that for every one mole of butane, 6.5 moles of oxygen are required to ensure that all the hydrocarbon is converted into carbon dioxide and water. If excess oxygen is present, complete combustion occurs. During incomplete combustion, the products carbon monoxide (CO) and carbon (soot) may be produced as well as water.Consider the combustion of one mole of methane (natural gas) in decreasing amounts of oxygen2CH4 (g) + 4O2 (g) 2CO2 (g) + 4H2O (g)
2CH4 (g) + 7O2 (g) CO2 (g) + CO (g) + 4H2O (g)
22CH4 (g) + 5O2 (g) CO (g) + C (s) + 4H2O (g)
2
Most school science laboratories use methane as a fuel for Bunsen burners. In a Bunsen burner, methane gas supplied from the gas mains or a cylinder enters at the base of the burner. Air is drawn through an adjustable air hole and the gas mixture burns at the top of the barrel. When the air hole at the base of a Bunsen burner is fully open, complete combustion occurs, carbon dioxide and water are produced, and the flame has a blue, luminous appearance. When the air hole is closed, less oxygen mixes with the methane and incomplete combustion occurs. Consequently the methane burns, producing carbon in the form of soot, and water. in these conditions the flame has a characteristic yellow colour.
216. Describe combustion in terms of slow, spontaneous and explosive reactions and explain the conditions under which these occur
The combustion reactions we use in everyday life proceed at very different rate. We have:
217. Slow combustion as in slow combustion stoves where large lumps of fuel

(coal, coke or wood) take many hours to burn218. Fast combustion such as burning methane, LPG, kerosene, or heating oil
in stoves or heating appliances or of powdered coal in power stations, and219. Explosive combustion as in the cylinders of petrol and diesel engines in
vehicles
All of the chemical reactions involved in these combustion processes are spontaneous ones. This means that once started (ignited) they proceed without further assistance and continue to go until all of the fuel is used up.Slow combustion occurs when we use big lumps of fuel and limit the supply of air. This means that burning occurs only on the surface of the big lumps and its speed is controlled by the limited supply of air.For fast combustion in power stations, coal is ground into very small particles that are sprayed into a plentiful of air: there is a large surface area of fuel exposed to an excess of oxygen and there is good mixing to stop oxygen concentrations becoming depleted near the surface of the particles. In other fast combustion appliances, gaseous fuel or vaporised liquid fuel is mixed with excess air: the gaseous nature of the mixture and the high temperature ensure that fuel is always in contact with oxygen and so combustion proceeds rapidly.In petrol engines a spark is used to ignite a heated mixture of petrol and air. In diesel engines liquid fuel is injected and vaporised into an excess amount of heated air. In both cases the conditions used are such as to promote very rapid reaction. An explosion is just an extremely rapid reaction- one that goes to completion within a few microseconds.Changes in conditions lead to changes in the rates of the reactions.
220. Explain the importance of collisions between reacting particles as a criterion for determining reaction rates
The first step in a chemical reaction is thought to involve a collision between the reactant particles. This idea is part of the collision theory of reaction rates. The collision theory assumes that if particles are to react, they must first undergo an appropriate collision.According to the kinetic theory, the particles in a gas are in a continuous state of random straight- line motion. While most of the particles have energies that are close to the average for all the particles in the system, a small number have energies much lower or much higher than the average. Because of this range of kinetic energies, collisions between HI molecules will occur with different energies. A collision between reactant molecules does not necessarily mean that a reaction will take place. In fact, most collisions do not bring about a reaction. The collision theory requires that for a collision between reactant particles to lead to a chemical reaction, the following conditions must be fulfilled:
221. The molecules must collide with sufficient energy to disrupt the bonds of the reactant molecules.
222. The molecules must collide with an orientation that is suitable for the breaking of some bonds and the formation of others.

For a reaction to occur between reactant molecules, they must collide with a certain minimum energy. Unless this minimum collision energy is exceeded, the colliding molecules will simply rebound and move away form each other. The minimum energy that is required for a collision to result in a reaction is known as the activation energy for the particular reaction. Some reactions have relatively low activation energies and so react at a significant rate at room temperature. For these reactions, a noticeable reaction occurs as soon as the reactants are mixed. For example, a piece of sodium metal is added to water at room temperature produces a violent reaction almost instantaneously. Other reactions occur to an almost insignificant extent at room temperature. In such reactions the activation energy is so high that it is very unlikely that reactant molecules will collide with sufficient energy to undergo reaction. For example, methane will not react with oxygen unless the mixture is ignited. There is a relatively high activation energy for the reaction between methane and oxygen. Despite the fact that under standard conditions there would be in the order of 1010 collisions per second between reactant molecules, virtually none of these would have sufficient energy for a reaction to take place.
Nature of the reactantsIn general, if other factors affecting reaction rates are equal, slower reactions will have higher activation energies than faster reactions. The different activation energies for different reactions are related to the ease with which bond- breaking and re- forming processes occur. High activation energies are often associated with reactions in which strong bonds have to be broken. For example, the low reactivity of nitrogen gas (N2) is related to the large amount of energy needed to break the N N triple bond.
ConcentrationOne of the major assumptions of the collision theory is that molecules must collide before they react. The effect of increasing the concentration of a reactant in solution is to increase the rate at which reactant molecules collide with one another. If a greater number of collisions occur per unit of time, the reaction rate will increase. Although the same proportion of these collisions will be successful, the greater rate of collisions results in a greater number of successful collisions. The same argument can be applied to reactions involving gases. Increasing the pressure of reactant gases increases the rate of collisions between the molecules and hence the rate of reaction.
The extent of the increase in reaction rate which occurs when the concentration of reactants is increased depends on the reaction involved. Often the realationships is a fairly simple one. For example, in the reaction
H2 (g) + I2 (g) 2HI (g)
Doubling the pressure of H2 (g) doubles the rate of reaction. For the reaction2HI (g) H2 (g) + I2 (g)
doubling the pressure of HI(g) quadruples the rate.
Surface AreaIf the surface area of a solid or liquid reactant is increased, more of the reactant molecules are exposed to collision. Subdividing a piece of solid reactant increases its

surface area. Since molecules must collide before they react, increasing the surface area can lead to an increased rate of reaction. Solid reactants are often powdered to increase the rate of reaction. When liquid reactants are used, the liquids are frequently used as sprays or subjected to vigorous agitation to ensure that a large surface area is available for reaction. (SEE TEMPERATURE AFFECTING REACTION RATES AND CATALYSTS LATER ON)
223. Explain the relationship between temperature and the kinetic energy of particles
The temperature of a reacting system has a significant effect on the rate of reaction. An increase of 10oC above room temperature can lead to a two- or three- fold increase in reaction rate. An increase in temperature increase the average kinetic energy of the reacting molecules and, more importantly, changes the distribution of molecular kinetic energies.The increased velocities of the molecules lead to a greater rate of collision and hence a greater rate of reaction. However, the increase in reaction rate due to the increased rate of collision is fairly small.
The main reason that an increase in temperature increases reaction rate is because of its effect on the distribution of molecular kinetic energies. As the temperature is raised, a greater proportion of reactant molecules have sufficient kinetic energy to supply the activation energy needed for reaction. This means that a greater proportion of molecular collisions will be successful collisions. As a result, the rate of the reaction is increased. (SEE GRAPHS)
224. Describe the role of catalysts in chemical reactions, using a named industrial catalyst as an example
A dramatic increase in the rate of certain chemical reactions can be produced by the use of substances known as catalysts. A catalyst is a substance, or mixture of substances, that increase the rate of a chemical reaction without being permanently consumed in the reaction. For example, manganese (4) oxide (MnO2) acts as a catalyst to increase the rate of decomposition of hydrogen peroxide (H2O2). In the absence of a catalyst, H2O2 decomposes into oxygen and water at a very slow rate.
2H2O2 (aq) O2 (g) + 2H2O (l) (very slow)
The addition of a small amount of MnO2 causes the H2O2 to bubble vigorously due to the rapid production of O2 (g). The mass of MnO2 is the same before and after the reaction, showing that the catalyst is not consumed.
2H2O2 (aq) MnO2 O2 (g) + 2H2O (l) (fast)
The catalyst is written above the arrow to show that although it is not consumed, it play

as essential role in the reaction.In industry, a wide variety of processes employ catalysts to increase the rates of reactions that would be uneconomically slow without them. For example, the oxidation of sulfur dioxide to sulfr trioxide in the manufacture of sulfuric acid uses a platinum (Pt) or vanadium pentoxide (V2O5) catalyst. Many chemical processes that occur in nature, such as photosynthesis and digestion, only proceed at a significant rate because of the action of catalysts. In many of these processes, enzymes are involved. Enzymes are biochemical catalysts that are usually highly specific in their function. This means that they act to catalyse one type of chemical reaction but have no influence on other reactions.
225. Explain the role of catalysts in changing the activation energy and hence the rate of chemical reaction
A catalyst provides an alternative reaction pathway to that available when the reactants alone are used. This different pathway has a lower activation energy. The catalyst allows the reaction to occur in such a way that less collision energy is required for reaction to take place. As a result, a greater proportion of reactant collisions will be successful and the reaction rate will be greater than without a catalyst. It is important to note that the activation energies for both the forward and reverse reactions are decreased by the action of a catalyst. The rates of both the forward and reverse reactions are therefore increased by the use of a catalyst.Catalysts are extremely important in industry because they help make reactions possible that would normally be slow or require large amounts of heating to achieve. The use of catalysts often allows reactions to be carried out at much lower temperatures, thereby using less energy and reducing costs.

DEFINITIONS

Homogeneous- means of uniform composition throughout eg water, sugar, aluminium, petrol, whisky.
Heterogeneous- means having non- uniform composition where we can recognise small pieces of the material which are different from other pieces eg strawberry jam, wood, water with ice.
Impure substance- is one substance contaminated with small amounts of one or more other substances.
Element- is a pure substance consisting of one kind of matter particle or atom which cannot be further decomposed into simpler substances by chemical means.
Compound- is a pure substance which can be decomposed into simpler substances, for example into elements. Compounds can be classified as covalent molecular, ionic or covalent network depending on the arrangement of the atoms within the compound.
226. A compound is made of 2 or more elements 227. Always has the elements present in the same ratio by mass 228. Has properties that are quite different from those of the elements that
make it up.
Law of conservation of matter- Matter can be neither created nor destroyed, but merely changed from one form to another.
Mixture- A mixture is composed of 2 or more substances. A mixture can be separated into 2 or more pure substances by physical or mechanical means such as filtering, boiling or using a magnet or tweezers. May be homogeneous (tap water, air) or heterogeneous (fruit cake, concrete). The physical properties of a mixture eg Boiling point, density, solubility and melting point reflect the properties of the components of the mixture. Its properties can change as the relative amounts of the substances present are changed. Has a variable composition, that is the relative amounts of each pure substance present can be varied. Eg sea water, coffee, milk, petrol, whisky, brass, and ‘silver’ coins.

Pure Substance- cannot be separated into 2 or more substances by physical or mechanical means. Is homogeneous (crystals of sugar, piece of copper). Has properties (characteristics) such as appearance, colour, density, melting and boiling points, which are constant throughout the whole sample. Has properties which do not change regardless of how it is prepared or how many times it is subjected to purification procedures. Has a fixed composition, no matter how it is made or where it comes from. Eg table salt, sugar, copper, aluminium, diamond, gold, polythene, and alcohol.
Atmosphere- The atmosphere is the body of air which surrounds our planet. Most of our atmosphere is located close to the Earths surface where it is most dense. It is about 200- 300 Km thick.
Hydrosphere- The hydrosphere is composed of all of the water on or near the earth. This includes the oceans, rivers, lakes, ground water and even the moisture in the air.
Lithosphere- The lithosphere is the solid, rocky crust covering the entire planet. It includes the crust and the top portion of the mantle. This crust is inorganic and is composed of minerals.
Biosphere- the portion of Earth inhabited and used by living matter; the biosphere consists of the atmosphere, hydrosphere, and lithosphere.
Distillate- The liquid collected from a distillation.
Volatile- Liquid's or solids ability to be converted into vapour. If something is extremely volatile it has a high tendency to vapourise or become gaseous.
Immiscible liquids- 2 liquids are said to be immiscible if, when they are mixed they do not form a homogeneous liquid but instead stay as drops of one liquid dispersed through the other liquid (usually forming two separate layers of liquids)
Miscible liquids- Liquids which mix to form a homogeneous liquid are said to be immiscible.
Suspension- A suspension is a dispersion of particles through a liquid with the particles being sufficiently large that they settle out on standing.
Solution- A solution is a homogeneous mixture in which the dispersed particles are so small (molecules or ions) that they never settle out and cannot be seen by a microscope.
Solute- The substance which is dissolved in a solution.
Solvent- The liquid which does the dis solving.
Changes of state- changes from solids to liquids to gases and vice versa.

Sublimation- change of state from solid to gas and from gas to solid.
Melting Point- The melting point of the substance is the lowest temperature at which the solid changes to a liquid.
Freezing point- Highest temperature at which a liquid can be converted to a solid.
Boiling- process of converting a liquid to a gas by heating the liquid until visible bubbles of vapour form throughout the liquid and quickly rise to the surface.
Boiling point- the lowest temperature at which the boiling process occurs.
Normal boiling point- the boiling point at standard atmospheric pressure.
Gravimetric analysis- Determining the quantities (masses) of substances present in a sample is called gravimetric analysis.
Semi- metals- elements that have properties intermediate between metals and non- metals.
Atom- An atom is the smallest particle of an element which is still recognisable as that element.
Molecule- A molecule is the smallest particle of a substance that is capable of separate existence.
Symbols- used for elements
Formulae- combination of symbols used for compounds are called formulae
Valence- the measure of an elements combining power.
Electrolysis- decomposing a substance with an electric current
Direct Combination reactions- in addition to reactions in which one compound is decomposed into 2 or more substances (elements or compounds), there are many reactions in which a compound is formed from its elements. These are called direct combination reactions.
Physical properties- properties of substances that relate to physical changes, such as melting and boiling points, are called physical properties. Eg appearance, density, electrical conductivity, and hardness.
Chemical properties- properties that relate to the chemical reactions that substances undergo are called chemical properties. Eg ease of decomposition by heat, effect of light, and reactivity with other substances such as oxygen, chlorine and sulphur.

Reactants- the starting substances in which are involved in the reaction.
Products- the substances that are formed from the reactants.
Chemical change- a change in which at least one new substance is formed is called a chemical change.
Covalent lattice solids/ covalent network solids- are solids in which the covalent bonding extends indefinitely throughout the whole crystal.
Intermolecular forces- forces between pairs of molecules
Covalent molecular substances- substances that are made up of simple covalent molecules.
Ionic bonding- That type of chemical bonding which involves the outright transfer of electrons from one atom to another.
Covalent bonds- are formed between pairs of atoms by the atoms sharing electrons.
Empirical formulae- Such formulae that give the ratio by atoms of elements in a compound rather than the actual number of atoms in a molecule are called empirical formula. Formulae for ionic compounds are therefore always empirical formulas (because there are no molecules)
Ions- positively or negatively charged particles.
Valence electrons- the electrons in the highest energy level.
Cation- positively charged particle.
Anion- negatively charged particle.
Valence shell- outermost energy level.
Electron configuration- the arrangement of electrons in energy levels.
Mass number- is the number of protons plus neutrons in the nucleus of an atom of the species concerned.
Atomic number- of an element is the number of protons in the nucleus of an atom of that element.
Family of elements- a group of elements which differ from their nearby noble gases in the same way will have very similar chemical properties.

Transition metals- arise from converting a semi- filled level to a completely filled level (8 to 18 electrons)
Proton- a proton is a small positively charged particle having a mass approximately equal to a hydrogen atom and a charge equal in magnitude (but opposite in sign) to that of an electron.
Neutron- A neutron is a small neutral particle which has the same mass as a proton
Electron- An electron is an extremely small negatively charged particle with a mass of approximately 1/2000 of the mass of a hydrogen atom
Electron cloud- many electrons moving randomly through the volume surrounding the nucleus that we use the term electron cloud to describe it.
Monatomic molecules- The noble gases exist as independent atoms so they consist of monatomic molecules.
Diatomic molecules- pairs of atoms stuck together to form molecules
Isotope- Atoms of the same element with different number of neutrons in the nucleus.
Copper age- 5000- 3000BC
Bronze age- 3000- 1000BC
Iron age- from 1000BC
Rusting- susceptible to corrosion
Ore- if the mineral is present in sufficient quantity to make the mining and extraction of the metal economically viable, it is called an ore.
Tailings- Old Mine dumps
Metallurgy- the total process involving the 4 steps of converting ore to refined metal
Ionisation energy- The ionisation energy of an atom or ion is defined as the amount of energy required to remove the most loosely bound electron from the atom or ion in the gaseous state.
Mole- A mole is defined as the amount of a substance that contains the same number of particles as there are atoms in exactly 12g of carbon-12.
Relative formula mass- Because ionic compounds do not contain molecules, the sum of the relative atomic masses of the atoms in the formula is called the relative formula mass.

Relative molecular mass- The relative molecular mass of a substance is the mass of a molecule of that substance compared with 1/12th the mass of an atom of carbon-12. Relative molecular masses have no units.
Electronegativity- The measure of attraction that element have for electrons. Resultantly, Fluorine has the highest electronegativity since it has the strongest attraction for electrons
Oxidised- The substance that loses electrons
Reduction- The substance that gains electrons
Half- equations- used in oxidation- reduction reactions with one part representing the oxidation reaction and the other part representing the reduction reaction. Each part is called a half equation.
Reducing agent- a substance that causes the reduction of another substance
Oxidising agent- A substance that causes the oxidation of another substance
Tailings- Old mine dumps
Molar mass- a mole of any substance has a mass equal to its relative atomic mass, relative molecular mass or relative formula mass expressed in grams. This is known as the molar mass.
Stoichiometric ratio- the ratio of moles of different substances in a balanced chemical equation
Theoretical Yield- The theoretical yield in a chemical reaction is the quantity of product predicted from the chemical equation when known quantities of reactants undergo the reaction.
Water of crystallisation or water of hydration- water that become bound to a pure substance during crystallisation is called water of crystallisation
change of state- changes from solid to liquid to gas and vice versa are called changes of state
decant- process of carefully separating a liquid from a solid by pouring off the liquid and leaving the solid undisturbed at the bottom of the container
density- density is defined as mass per unit volume
distillation- Distillation is a method of separating liquids from solutions or of purifying one liquid. It involves boiling the material and condensing the resulting vapour back to a liquid in a different part of the apparatus.

evaporate to dryness- Evaporate to dryness means heating a solution in an evaporating basin to drive off all the solvent (the liquid).
evaporation- In evaporation the liquid is heated to a temperature below its boiling point (or just let sit in an open vessel on the laboratory bench) so that some of the particles escape from the surface of the liquid into the air and get blown away. Evaporation is a slower process than boiling
filtrate- The liquid or solution which passes through the filter paper is called a filtrate
filtration- separating solids and liquids by passing the mixture through a filter paper. The liquid passes through while the solid remains on top of the filter paper.
fractional distillation- To separate liquids by distillation when their boiling points are fairly close together.
groups- the vertical columns on the Periodic table
periods- The horizontal rows on the Periodic Table
main- group elements - All the elements from group 1 to 8, excluding the transition elements are called main- group elements.
noble gases- Helium, Neon, Argon, Krypton, Xenon and Radon are called noble gases because they form virtually no compounds.
Periodic Table- A chart which organises all the elements into groups with similar properties
Sedimentation- sedimentation is the process in which solids settle to the bottom of a container
Separating funnel- Pear- shaped piece of apparatus that tapers down to a narrow tube just above the stopcock.
Sieving- Process used to separate mixtures in which the particles of the different substances have different sizes.
States of matter- Whether the substance is a liquid, solid or gas
Transition elements- The ten groups of elements between Group 2 and 3 are called transition elements
Vaporisation- Vaporising can be achieved by either boiling the liquid or allowing it to evaporate

chemical bond- Bonds involving the sharing of electrons that hold atoms together
delocalised electrons- Electrons that no longer belong to particular atoms
discrete energy levels- The energy levels that electrons exist in when surrounding an atom
electron- dot diagram- These are symbols or formulae of elements or compounds with the valence electrons shown as dots
ionic lattice- ionic lattices are ionic solids. They are called ionic lattices because they consist of an infinite orderly array of particles joined together by electrostatic attraction.
lattice- An infinitely orderly array of particles
metallic bonding- metals consist of an orderly three- dimensional array of positive ions held together by a mobile sea of delocalised electrons.
nucleus- consists of the bulk of the mass of the atom and carries positive electrical charges.
stable electron configurations- The electron configuration of the noble gases are extremely stable because these gases are unreactive. Stable electron configurations are achieved by elements reacting with other elements in order to obtain a stable octet electron configuration like the noble gases.
balanced equation- A chemical equation must have the same number of atoms of each element on each side of the arrow
binary compound- Compounds consisting of 2 elements only
chemical equation- Equation used to describe what happens in chemical reactions
decomposition reaction- The break down of a compound into 2 or more other substances.
electrodes- The conductors which introduce an electric current to a liquid or solution are called electrodes
physical change- A change in which no new substance is formed is called a physical change
symbolic equation- Equations which use symbols and formulae for the elements and compounds involved.
word equation- Equation used to describe what happens in a chemical reaction using

words not chemical formulae or symbols
Activity series- Using the reactivity of metals with oxygen, water, and dilute acids to draw up a list of the metals in order of decreasing reactivity. This is called an activity series.
Alkali- An alkali is a soluble base
Alloy- An alloy is a homogeneous mixture of a metal with one or more other elements
Base- A base is a compound which contains the oxide or hydroxide ion or which in solution for the hydroxide ion
Complete ionic reaction- An equation that shows all the ions involved in the solutions used for the reaction
Electrical conductivity- electrical conductivity is the current passing through a 1 metre cube of the substance when a voltage of exactly 1 volt is applied across opposite faces of the cube: it is measured in megohm-1m-1
Froth flotation-
Half- reaction- Half reactions or equations are reactions which describe the oxidation and reduction processes separately in terms of electrons lost or gained
Hardness- The Brinell Hardness Number is based upon the size of the indentation made by forcing a hard sphere into the surface of the material. Another measure of hardness is the mohs scale of hardness with talc given a value of 1 and diamond 10. A substance with a higher number will be able to scratch a substance with a lower number.
Ionisation- The process of neutral species (atoms or molecules) undergoing reaction to form ions is called ionisation
Mineral- A mineral is a pure (or nearly pure) crystalline compound that occurs in the Earth’s crust.
Net ionic equation- An equation that shows the actual ionic species that undergoes change in the reaction.
Non- renewable resource- Resources formed when the Earth was formed and there is no way of forming any more of them
Oxidation- means loss of electrons
Recycling-
Redox reaction- In normal chemical reactions there are no overall loss or gain of

electrons. Hence oxidation and reduction occur simultaneously in complete chemical reactions. These reactions are called reduction- oxidation reaction, (redox reactions)
Reduction- means gain of electrons
Smelting- The term smelting is used for the extraction of a metal by heating substances to sufficiently high temperatures to produce a molten material from which the metal can be obtained
Spectator ions - Ions that do not undergo any change during a reaction are called spectator ions.
Steel- Many of the alloys in common use where the predominant metal is iron
Tensile strength- is a measure of how well a material resists bending, twisting or stretching and indicates the suitability of the material for structural purposes.Tensile strength is measured by stretching a sheet or rod of the material of known cross- sectional area until it breaks: the measured force needed to break the sample divided by the original cross- sectional area of the sample is the tensile strength. It has the units newton per square metre (N m-2) which is a pascal (Pa), the unit for air pressure
Thermal conductivity- thermal conductivity is the energy transmitted per second through a 1 metre cube of the substance when there is a 1oC temperature difference across opposite faces: it is measured in J s-1 m-1 K-1
Atomic weight - The relative atomic mass (or atomic weight) of an element is the average mass of the atoms present in the naturally occurring element relative to the mass of an atom of the carbon- 12 isotope taken as exactly 12
Avogadro’s constant- The Avogadro constant (for which we use the symbol NA) is the number of atoms in exactly 12 grams of the carbon- 12 isotope. NA = 6.022 x 1023 particles per mole. It is the basis for the number of molecules in a mole.
Avogadro’s hypothesis- Equal volumes of all gases, measured at the same temperature and pressure, contain equal numbers of molecules.
Dalton’s atomic theory- The three postulates of Dalton’s atomic theory were1) Matter is composed of tiny indivisible particles called atoms2) All atoms of the one element are identical, but different from the atoms of
all other elements.3) Chemical reactions consist of combining, separating or rearranging atoms
in simple whole number ratios
Formula weight- The relative formula mass (or formula weight) of a compound is the sum of the atomic weights of the atomic species as given in the stated formula of the compound

Gay- Lussac’s law of combining volumes- The ratios of the volumes of gases involved in a reaction, if measured at the same temperature and pressure, are expressed by small, whole numbers.
Molecular formula- The molecular formula of a compound is the formula which tells us how many of each type of atom are present in a molecule of the compound
Molecular weight- The relative molecular mass (or molecular weight) of a compound is the mass of a molecule of the compound relative to the mass of an atom of the carbon- 12 isotope taken as exactly 12. The molecular weight of a compound is the sum of the atomic weights of the atoms as given in the molecular formula
Percentage yield- The percentage yield of a chemical reaction is the amount of product obtained expressed as a percentage of the amount expected from the chemical equation
Relative atomic mass- The relative atomic mass (or atomic weight) of an element is the average mass of the atoms present in the naturally occurring element relative to the mass of an atom of the carbon- 12 isotope taken as exactly 12
Stoichiometry- The study of quantitative aspects of formulae and equations is called stoichiometry. The calculations involved are called stoichiometric calculation.
Atomic radius- the radius of an atom of a particular element
Mendeleev’s periodic law- Properties of the elements vary periodically with their atomic weights
Newlands’ law of octaves- when the elements were arranged in order of increasing atomic weight, ‘the eighth element starting from a given one is a kind of repetition of the first like the eighth not in an octave of music’
Periodic table, groups and periods-
Picometre- 1 picometre (pm) = 10-12 metre
Successive ionisation energies- The first ionisation energy of an element is the energy required to remove one electron from the neutral atom. The second ionisation energy is the energy required to remove a second electron. The second ionisation energy is always greater than the first.
The current periodic law- Properties of the elements vary periodically with their atomic numbers
Adhesion- Adhesion is the force with which a substance sticks to some other substance

Anhydrous- The term anhydrous is used to describe forms of salt which do not contain water of crystallisation
Capillary attraction- If a small diameter tube (say, 1 or 2 mm) is dipped into a liquid, the liquid rises up the tube if the liquid wets the surface of the tube. Small diameter tubes are called capillaries. This process is called capillary attraction. Adhesive forces draw the liquid up the walls of the tube until the weight of the liquid column being supported just balances the adhesive forces upwards. For capillary attraction to be noticeable the tube needs a diameter of less than about 5mm. A consequence of cohesion and adhesion is capillary attraction
Cohesion- Cohesion is the force with which a substance sticks together.
Diffusion- Diffusion is a mixing process in which particles move from a region where their concentration is higher to one where their concentration is lower
Electric Dipole- The occurrence of a positive charge and an equal negative charge separated by some fixed distance is called an electric dipole.
Dipole- dipole interactions-
Dispersion forces- The weak forces of attraction between all atoms or molecules arising from temporary dipoles are known as dispersion forces.
Heteroatomic- Molecules such as HCl, H2O and NH3 where the electron pairs are unevenly shared
Hydrates- salts which contain water of crystallisation are called hydrates
Hydrogen bonds- Hydrogen bonds are particularly strong types of polar interactions. They result when a hydrogen atom, bound to one of the three atoms, F, O or N can become attached to another one of these atoms in another molecule. Water and ammonia display hydrogen bonding.
Ionisation reactions- reactions when covalent molecular substances react with water to form ions
Meniscus- The curvature of the surface of a liquid near the solid (e.g. measuring cylinder) at the top, where the water is at its highest point. Concave up for water, and convex up for mercury
Non- polar solvent- A non- polar solvent is a liquid which consists of non- polar molecules. Hexane (C6H14) and carbon tetrachloride (CCl4) are non- polar solvents
Osmosis- Osmosis is a specific form of diffusion, involving the movement of water from regions where it is highly concentrated to other regions through a semi- permeable

membrane such as a cell membrane.
Polar covalent bond - Covalent bonds in which the electrons are unequally shared are called polar covalent bonds
Polar molecule- Polar molecules are ones which have a net dipole
Polar solvent- Water it a polar solvent. This is because it consists of polar molecules
Semi- permeable membrane- semi- permeable membrane means that some substances, usually small molecules or ions, are able to pass through small pores or holes in the membrane while large molecules cannot.
Surface tension- Surface tension is a measure of the resistance of a liquid to increasing its surface area. The higher the value, the greater the tendency of the liquid to form a spherical drop rather than spread out as a thin film.
Viscosity- Viscosity is a measure of the resistance of a liquid to being poured or to flowing through a tube. The higher the viscosity, the less easily the liquid flows. Honey and motor oil have much higher viscosities than water
Concentration- The concentration of a solution is the amount of solute present in a specified amount of solvent or solution
Dilution- Dilution involves lowering the concentration of a substance in a solution from a higher concentration to a lower concentration.
Dynamic equilibrium- Dynamic equilibrium occurs when both the forward and reverse reactions are occurring at equal rates so that there is no overall change in terms of the amount of products and reactants remaining
Endothermic- Processes which absorb heat are called endothermic
Exothermic- Processes which release heat are called exothermic
Forward reaction- The left to right reaction (left hand side to right hand side of the equation) is called the forward reaction.
Heat of solution- heat of solution is the enthalpy change when a solute dissolves in a solvent to form a solution.Heat of solution = heat absorbed during dissociation – heat released during hydration
Heavy metal pollution- Heavy metals are the transition metals plus lead. Heavy metal pollution is when these metals are present in high concentrations
Molarity- The molarity of a solution is the number of moles of solute per litre of solution

Parts per million (ppm)- Grams of solute per million grams of solution
Per cent by weight- mass of solute per 100grams of solution
Pollution- Pollution is the presence of harmful or undesirable substances in the environment at concentrations significantly greater than those in the neutral (or unpolluted) environment
Precipitation reaction - A solid produced by reaction between two clear solutions is called a precipitate. Such reactions are called precipitation reactions
Quantity of heat- Amount of heat (or quantity of heat) is different from temperature. The amount of heat energy is proportional to the mass of the substance involved
Reverse reaction- The right to left reaction (right hand side to left hand side of the equation) is called the reverse reaction
Reversible reaction- A reaction that is able to go in either direction (the forward reaction or the reverse reaction) is called a reversible reaction.
Saturated solution- A saturated solution is one in which no more of the particular solute can be dissolved in a given quantity of the solvent at the specified temperature.
Specific heat capacity- The specific heat capacity of a substance is the amount of heat required to raise the temperature of unit mass of the substance through one degree Celsius (or through one Kelvin)
Solubility- The solubility of a substance in a particular solvent is the concentration of its saturated solution at the specified temperature. It is the maximum amount of the substance that will dissolve in a given amount of the solvent
Temperature- Temperature is a measure of the degree of hotness or coldness of an object or substance. The hotter the object, the higher its temperature
Thermal equilibrium- If two objects or samples of material are brought into contact, heat will flow from the hot object to the cold one until the temperature of the two objects is equal. When the temperature is uniform throughout both objects or samples of material, we say that thermal equilibrium has been reached.
Thermal pollution- Thermal pollution is the discharge into a river or lake of quantities of hot water that are large enough to increase significantly the temperature of the water body. A 2 to 5oC increase can be ‘significant’.

Unsaturated solution- A solution which contains less than the maximum amount of solute is called an unsaturated solution
Alkanes- Hydrocarbons in which all the bonds are single bonds are called alkanes. CnH2n+2
Alkenes- Hydrocarbons which contain a double bond between a pair of carbon aroms are called alkenes. CnH2n
Alkynes- There is a homologous series of hydrocarbons containing triple bonds. They are called Alkynes. CnH2n-2
Allotropes-Allotropes are forms of the one element (in the same physical state) which have distinctly different physical properties (colour, density, hardness, electrical conductivity). The reason allotropes have different properties is that the atoms are joined or packed together in different ways to form molecules or crystals
Ball- and- stick models- represent molecules as balls (atoms) held together by sticks (bonds)
Carbohydrate- Carbohydrates are compounds of carbon, hydrogen, and oxygen. Common carbohydrates are glucose, fructose, sucrose, starch, glycogen and cellulose
Condensed structural formula- A condensed structural formula is a ‘cross’ between a molecular formula and a full structural formula. It provides enough detail for a full structural formula to be written if required, but writes common groups of atoms in molecular form
Double bond- 2 shared pair of electrons between a pair of atoms
Fossil fuel- Fossil fuels are substances which were formed by the action of high temperature and high pressure upon decaying plant and animal matter over millions of years.
Functional group- Term used to describe the centre of reactivity in a carbon compound. In alkenes and alkynes, the functional groups are the double and triple bonds. This is because that is what makes them very reactive, the fact that the double or triple bond can break and react with something else makes them very reactive. The functional group is ‘where the action is’. Similarly, when a halogen atom, OH group or NH2 group replaces a hydrogen atom in an alkane, the molecule becomes much more reactive, again with the reactivity centred around the halogen atom, the OH group or NH2 group. When a double or triple bond is introduced, the molecule becomes much more reactive, but the reactivity is associated with the double or triple bond, not with the rest of the molecule.
Homologous series- A family of compounds which can be represented by one general

molecular formula is called a homologous series
Hydrocarbons- Compounds of carbon containing only carbon and hydrogen are called hydrocarbons
Isomers- Isomers are sets of different compounds which have the same molecular formula but different structural formulae
Photosynthesis- Photosynthesis is the process in which plants use solar energy to convert carbon dioxide from the air and water from the ground into carbohydrates such as glucose, sucrose, starch and cellulose. Solar energy is converted into chemical energy by photosynthesis.
6CO2 (g) + 6H2O (l) + energy C6H12O6 (aq) + 6O2 (g)
Respiration- Carbohydrates in plants are the energy source (food) for animals, including humans. In cellular respiration, the stored chemical energy is made available to the animal:
C6H12O6 + 6O2 6CO2 + 6H2O + energy
The amount of energy released during respiration is the same as was absorbed during photosynthesis, namely 2830kJ per mole of glucose used.
Saturated and unsaturated hydrocarbons- Alkenes and Alkynes are called unsaturated hydrocarbons. This mean unsaturated with respect to hydrogen, because it is possible to attach more hydrogen to the molecule by breaking the double or triple bond and forming single bonds to extra hydrogen atoms (for example to convert C2H2 or C2H4 to C2H6). Alkanes are called saturated hydrocarbons, because they contain the maximum number of hydrogen atoms that the particular carbon skeletons can accommodate
Single bond- one shared pair of electrons between a pair of atoms
Space- filling models- the best description of the physical appearance of molecules is given by so- call space- filling models. However these models do not show the nature of the chemical bonding very clearly.
Straight- chain hydrocarbons- molecules with the carbon atoms strung together in a long chain: each C atom is joined to no more than two other C atoms
Branched chain hydrocarbons- Molecules with some carbon atoms joined to three or four other carbon atoms
Cyclic hydrocarbons- molecules with some carbon atoms joined into rings of five or six carbon atoms with chains attached to the rings

Aromatic hydrocarbons- molecules that contain some six- membered rings with bonds that are not just the simple single C-C bonds of the other three classes
Structural formula- drawings in which dashes are used to indicate bonding pairs of electrons.
Triple bond- 3 shared pair of electrons between a pair of atoms
Activation energy- The activation energy, Ea, of a reaction is the minimum amount of energy reactant molecules must possess in order to drive the reaction in favour of the products.
Absorb- Stick on
Average rate of reaction- Alternatively the average rate of reaction over a small time interval is the change in concentration divided by the time taken for the change to occur. Reaction rate decreases as reaction proceeds
Catalyst- Substances which increase the rate of a reaction without undergoing permanent chemical change in the reaction are called catalysts
Combustion- Combustion is a process in which a self- sustaining chemical reaction occurs at temperatures above those of the surroundings. More simply, combustion is burning. Explosions are also forms of combustion.
Enthalpy change for a reaction- the change in enthalpy for a chemical reaction, is defined as the heat absorbed (per mole of specified reactant or product) when the reaction occurs at constant pressure. Change in enthalpy = enthalpy of products – enthalpy of reactantsFor endothermic reactions change in enthalpy is positiveFor exothermic reactions change in enthalpy is negative
Enzymes- Enzymes are biological catalysts. Enzymes are proteins which bring about important reactions in living plant and animal cells. Living organisms contain many enzymes; each catalyses a particular reaction.
Greenhouse effect- (global warming) Increasing atmospheric concentrations of carbon dioxide and some other gases such as methane result in more of the heat that Earth is radiating into space being trapped by the atmosphere. Consequently, average global temperatures are increasing. This is expected to cause detrimental climate changes and rising sea levels.
Heat of combustion- the heat of combustion of a substance is the heat liberated when one mole of the substance undergoes complete combustion with oxygen at a constant pressure of one atmosphere with the final products being carbon dioxide gas and liquid water

Heat of reaction- the heat of reaction is the amount of heat released or absorbed when a chemical reaction occurs.
Heterogeneous catalyst- A heterogeneous catalyst provides a surface on which the reaction occurs more rapidly than it does in the bulk of the reaction mixture. Finely divided nickel catalyses the reaction between unsaturated oils and hydrogen to form saturated fats (margarine). It is a heterogeneous catalyst. The reaction occurs between gaseous hydrogen and liquid oil on the surface of the solid nickel particles.
Heterogeneous reaction- Heterogeneous reactions involve species which occur at the interface between two phases or states
Homogeneous catalyst- Homogeneous catalysts work throughout the bulk of the reaction mixture (gas or solution). Nitrogen dioxide is a homogeneous catalyst for the reaction between sulphur dioxide and oxygen. As another example, hydrogen peroxide reacts fairly slowly with acidic iodide in aqueous solution to form iodine. Adding about 10-4 mol/L sodium molybdate to the reaction mixture makes the reaction very fast. The molybdate ion, MoO4
-1, is a homogeneous catalyst for this reaction
Homogeneous reaction- Homogeneous reactions involve species which are all in the same state
Ignition temperature- The ignition temperature of a fuel- air mixture is the minimum temperature to which the mixture (or portion of it) must be heated in order for combustion to occur
Particulates- Particulates are very small particles of solids and /or droplets of liquid. The particles or droplets are so small that they do not easily settle out of the air.
Rate of reaction- The rate of reaction is the rate of change of concentration with time. In other words: the rate of reaction at any particular time is the slope of the curve at that time (concentration- time graph, change in concentration over change in time).
Wick- A wick is a piece of absorbent string, rope or fabric which dips into a fuel to help the fuel burn
Related Documents


![notes NOTES ]” BACKGROUND](https://static.cupdf.com/doc/110x72/61bd44c661276e740b111621/notes-notes-background.jpg)