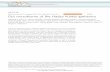
Gut microbiome associations with diet and medication usage in type 2 diabetes Thingholm, LB 1 , Rühlemann, MC 1 , Koch, M 2 ., Laucke, G 3 , Boehm, R 4 , Bang, C 1 , Heinsen, FA 1 , Frost, F 5 , Lerch, MM 5 , Homuth, G 6 , Kacprowski, T 6 , Lieb, W 7 , Laudes, M 8 , Huttenhower, C 9 , Franke, A 1 1) Institute of Clinical Molecular Biology, Christian-Albrechts-University of Kiel, Germany; 2) Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, USA; 3) UKSH ITSG, Lübeck, Germany; 4) Institut für Experimentelle und Klinische Pharmakologie, UKSH, Kiel, Germany; 5) Department of Medicine A, University Medicine Greifswald, Germany; 6) Department of Functional Genomics, Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Germany; 7) Institute of Epidemiology, Christian-Albrechts-University of Kiel, Germany; 8) Department of Internal Medicine I, University Hospital S.- H. (UKSH, Campus Kiel), Germany; 9) Biostatistics Department, Harvard T.H. Chan School of Public Health, Boston, USA INTRODUCTION The gut microbiome is essential for human health, and disturbed microbial communities have been associated with a broad range of medical conditions. However, it has proven difficult to identify detailed, disease-specific microbial features that are robust across populations. This limitation is partially due to the high variability of the gut microbiome, likely due to as-yet-uncaptured environmental influences. In the current study we evaluate the importance of medication and food supplements in relation to microbial studies of obesity and type 2 diabetes (T2D). See Figure 1 for overview of study cohorts. RESULTS We identified strong effects of commonly used medications as well as dietary components (minerals and vitamins). Five main drug classes showed strong effects on the microbial composition (Table 3). Similarly, we analyzed the effect of five commonly used food supplements, and found all to be significantly associated with either abundance or functional composition of the gut microbiome (q-value < 0.1). When we considered the correlation of selected nutrients and food groups (assessed by FFQ) with variance in gut microbial composition (BC dissimilarity) we found high-energy variables, and vitamins and minerals, most associated (Figure 2). However, age and gender were the variables most strongly associated to BC dissimilarity, while the overall variance explained was restricted to 1.44% for genera across lean non-diabetic samples, including 24 nutritional variables. CONCLUSION The associations of gut microbial features with food supplements and medication intake are of interest both as a source of confounding effects when studying the microbiome and health outcomes, and for their potential direct clinical relevance. Our findings thus emphasize the need to profile these environmental covariates, along with demographic and biometric factors, in population studies of health outcomes. Table 1: Five drug classes and food supplements evaluated for association with microbial composition. Linear discriminant analysis (LDA) with the first 10 principal components (PCoA, Bray Curtis dissimilarity, 16S rDNA data at genera level or metagenomic pathway abundance data) was used to evaluate the difference in microbial composition between users and non users of each drug class or food supplement. Raw and Benjamini-Hochberg (BH) adjusted p-values are shown for analyses on Northern German cohorts (PopGen and FoCus) and North-eastern German cohorts (SHIP). Contact details: Louise B. Thingholm, Phone IKMB: +49(0)431500-15172 | E-mail: [email protected] | LinkedIN profile: http://dk.linkedin.com/in/louisebruunthingholm Figure 2 (above): Association of age, gender and nutrition with gut microbiome composition. Panel (A) shows the association of age and gender with the first two unconditioned dbRDA axis (Capscale in R/Vegan) of gut microbiome relative abundance data. Axis labels show percentage variance explained calculated as axis eigenvalues proportion of total eigenvalues. Panels (C) and (D) show top nutrients and food groups associated with gut microbiome composition (Adonis in R/Vegan, . = 0.1-0.2; * = 0.05-0.1; ** = 0.01-0.05; *** ≤ 0.01). Age and gender associated significantly with the microbiome composition, while total energy intake showed limited association (panel A and B). The individual nutrition-derived variables explained limited variance in gut microbiome composition, compared to age and gender. The plot include non-diabetic lean samples with 16S rDNA data analyzed at genera level. T. indicate variables were daily intake is calculated from both nutrition and food supplements. BC: Bray-Curtis dissimilarity. Grebl tea: Grey and black tea. Sug,conf,cak,bui: sugar, confectionary, cakes and biscuits. Food Supplements Drug Classes PopGen & FoCus PopGen & FoCus SHIP p-value BH adjusted p-value p-value BH adjusted p-value p-value BH adjusted p-value Genera Genera Magnesium 2.60×10 -04 3.70×10-04 Antihypertensives 5.60×10 -05 1.40×10 -04 2.24×10 -07 1.12×10 -06 Vitamin and minerals 2.10×10 -04 3.70×10-04 Analgesics 2.08×10 -04 3.47×10 -04 1.87×10 -05 3.12×10 -05 Vitamin C 2.80×10 -04 3.70×10- 04 Antidepressants 4.80×10 -03 4.80×10 -03 3.33×10 -02 3.33×10 -02 Calcium 1.03×10 -03 1.00×10-03 Antiphlogistics 1.02×10 -03 1.28×10 -03 7.06×10 -05 8.83×10 -05 Antidiabetics 2.16×10 -05 1.08×10 -04 1.10×10 -05 2.75×10 -05 Pathways Pathways Magnesium 8.00×10 -04 3.20×10-03 Analgesics 7.12×10 -06 1.19×10 -05 Vitamin and minerals 5.60×10 -03 1.10×10-02 Antidepressants 1.18×10 -04 1.48×10 -04 Vitamin C 1.60×10 -02 1.60×10-02 Antihypertensives 2.20×10 -08 5.65×10 -08 Iron 1.30×10 -02 1.60×10-02 Antiphlogistics 0.00116 0.00116 Antidiabetics 2.26×10 -08 5.65×10 -08 Food Supplements Demographics Medication Nutrition Explained Variance in BC dissimilarity (R2) A B Age, gender and total energy intake D Food groups C Nutrients Figure 1 (above): Overview of study samples, health outcomes and factors of the gut microbial environment considered in the study. We analyzed 1,304 stool-derived amplicon profiles and 154 shotgun metagenomes from two samples from Northern Germany (cross-sectional cohort from PopGen & both cross sectional and clinical samples from FoCus). Findings for medication usage were validated in 880 samples from North-eastern Germany (SHIP). Three phenotypic groups were considered in the study: Lean non-diabetic, obese non-diabetic and obese type 2 diabetic (T2D) individuals. Environmental factors considered in the analysis include medication usage, general measures of health and fitness, nutrition and food supplement usage, and demographic factors. (Illustration by Sigurd Thingholm).

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Gut microbiome associations with diet and medication usage in type 2 diabetes
Thingholm, LB1, Rühlemann, MC1, Koch, M2., Laucke, G3, Boehm, R4, Bang, C1, Heinsen, FA1, Frost, F5, Lerch, MM5, Homuth, G6,Kacprowski, T6, Lieb, W7, Laudes, M8, Huttenhower, C9, Franke, A1
1) Institute of Clinical Molecular Biology, Christian-Albrechts-University of Kiel, Germany; 2) Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, USA; 3) UKSH ITSG, Lübeck, Germany; 4) Institut für Experimentelle und Klinische Pharmakologie, UKSH, Kiel, Germany; 5) Department of Medicine A, University Medicine Greifswald, Germany; 6) Department of Functional Genomics, Interfaculty Institute for Genetics and Functional Genomics, University Medicine Greifswald, Germany; 7) Institute of Epidemiology, Christian-Albrechts-University of Kiel, Germany; 8) Department of Internal Medicine I, University Hospital S.-
H. (UKSH, Campus Kiel), Germany; 9) Biostatistics Department, Harvard T.H. Chan School of Public Health, Boston, USA
INTRODUCTIONThe gut microbiome is essential for human health, and disturbed microbial communities have been associated witha broad range of medical conditions. However, it has proven difficult to identify detailed, disease-specific microbialfeatures that are robust across populations. This limitation is partially due to the high variability of the gutmicrobiome, likely due to as-yet-uncaptured environmental influences. In the current study we evaluate theimportance of medication and food supplements in relation to microbial studies of obesity and type 2 diabetes(T2D). See Figure 1 for overview of study cohorts.
RESULTSWe identified strong effects of commonly used medications as well as dietary components (minerals and vitamins).Five main drug classes showed strong effects on the microbial composition (Table 3). Similarly, we analyzed theeffect of five commonly used food supplements, and found all to be significantly associated with either abundanceor functional composition of the gut microbiome (q-value < 0.1). When we considered the correlation of selectednutrients and food groups (assessed by FFQ) with variance in gut microbial composition (BC dissimilarity) we foundhigh-energy variables, and vitamins and minerals, most associated (Figure 2). However, age and gender were thevariables most strongly associated to BC dissimilarity, while the overall variance explained was restricted to 1.44%for genera across lean non-diabetic samples, including 24 nutritional variables.
CONCLUSIONThe associations of gut microbial features with food supplements and medication intake are of interest both as asource of confounding effects when studying the microbiome and health outcomes, and for their potential directclinical relevance. Our findings thus emphasize the need to profile these environmental covariates, along withdemographic and biometric factors, in population studies of health outcomes.
Table 1: Five drug classes and food supplements evaluated for association with microbial composition. Linear discriminant analysis (LDA) with the first 10 principal components (PCoA, BrayCurtis dissimilarity, 16S rDNA data at genera level or metagenomic pathway abundance data) was used to evaluate the difference in microbial composition between users and non users of each drugclass or food supplement. Raw and Benjamini-Hochberg (BH) adjusted p-values are shown for analyses on Northern German cohorts (PopGen and FoCus) and North-eastern German cohorts (SHIP).
Contact details: Louise B. Thingholm, Phone IKMB: +49(0)431500-15172 | E-mail: [email protected] | LinkedIN profile: http://dk.linkedin.com/in/louisebruunthingholm
Figure 2 (above): Association of age, gender and nutrition with gut microbiome composition. Panel (A) shows the association of age and gender with the first two unconditioned dbRDAaxis (Capscale in R/Vegan) of gut microbiome relative abundance data. Axis labels show percentage variance explained calculated as axis eigenvalues proportion of total eigenvalues. Panels(C) and (D) show top nutrients and food groups associated with gut microbiome composition (Adonis in R/Vegan, . = 0.1-0.2; * = 0.05-0.1; ** = 0.01-0.05; *** ≤ 0.01). Age and gender associatedsignificantly with the microbiome composition, while total energy intake showed limited association (panel A and B). The individual nutrition-derived variables explained limited variance in gutmicrobiome composition, compared to age and gender. The plot include non-diabetic lean samples with 16S rDNA data analyzed at genera level. T. indicate variables were daily intake iscalculated from both nutrition and food supplements. BC: Bray-Curtis dissimilarity. Grebl tea: Grey and black tea. Sug,conf,cak,bui: sugar, confectionary, cakes and biscuits.
FoodSupplements DrugClassesPopGen&FoCus PopGen&FoCus SHIP
p-value BHadjustedp-value p-value BHadjusted
p-value p-value BHadjustedp-value
Genera GeneraMagnesium 2.60×10-04 3.70×10-04 Antihypertensives 5.60×10-05 1.40×10-04 2.24×10-07 1.12×10-06Vitaminandminerals 2.10×10-04 3.70×10-04 Analgesics 2.08×10-04 3.47×10-04 1.87×10-05 3.12×10-05VitaminC 2.80×10-04 3.70×10-04 Antidepressants 4.80×10-03 4.80×10-03 3.33×10-02 3.33×10-02Calcium 1.03×10-03 1.00×10-03 Antiphlogistics 1.02×10-03 1.28×10-03 7.06×10-05 8.83×10-05
Antidiabetics 2.16×10-05 1.08×10-04 1.10×10-05 2.75×10-05
Pathways PathwaysMagnesium 8.00×10-04 3.20×10-03 Analgesics 7.12×10-06 1.19×10-05Vitaminandminerals 5.60×10-03 1.10×10-02 Antidepressants 1.18×10-04 1.48×10-04VitaminC 1.60×10-02 1.60×10-02 Antihypertensives 2.20×10-08 5.65×10-08Iron 1.30×10-02 1.60×10-02 Antiphlogistics 0.00116 0.00116
Antidiabetics 2.26×10-08 5.65×10-08
Food SupplementsDemographics
Medication
Nutrition
Explained Variance in BC dissimilarity (R2)
A B Age, gender and total energy intake D Food groups
C Nutrients
Figure 1 (above): Overview of study samples,health outcomes and factors of the gut microbialenvironment considered in the study.We analyzed 1,304 stool-derived amplicon profilesand 154 shotgun metagenomes from two samplesfrom Northern Germany (cross-sectional cohort fromPopGen & both cross sectional and clinical samplesfrom FoCus). Findings for medication usage werevalidated in 880 samples from North-eastern Germany(SHIP). Three phenotypic groups were considered inthe study: Lean non-diabetic, obese non-diabetic andobese type 2 diabetic (T2D) individuals.Environmental factors considered in the analysisinclude medication usage, general measures of healthand fitness, nutrition and food supplement usage, anddemographic factors. (Illustration by SigurdThingholm).
Related Documents