Gastrointestinal Tract Mucosal Immunity Wat Mitthamsiri, MD. Allergy and Clinical Immunology Unit Department of Medicine King Chulalongkorn Memorial Hospital

GI mucosal immunity
May 07, 2015
GI mucosal immunity
Presented by Wat Mitthamsiri, M.D.
March13, 2014
Presented by Wat Mitthamsiri, M.D.
March13, 2014
Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Gastrointestinal Tract Mucosal Immunity
Wat Mitthamsiri, MD. Allergy and Clinical Immunology Unit
Department of Medicine King Chulalongkorn Memorial Hospital

Outlines
• GI Tract structures and functions • GI immune system • GI immune tissue • Innate immunity in GI tract • Adaptive immunity in GI tract

GI tract immunity: Importance
• Largest organ exposed to external antigens • Multifunctional:
– Mucosal barrier – Absorptive surface – Regulation to balance immune reactions
against foreign antigens – Fostering the symbiotic, commensal microbiota – Maintain state of tolerance to benign antigens – Maintain appropriate response to pathogenic
insults

GI TRACT STRUCTURES AND FUNCTIONS

Overview

Overview
http://cnx.org/content/m46506/latest/2402_Layers_of_the_Gastrointestinal_Tract.jpg

Esophagus: Overview
http://www.highlands.edu/academics/divisions/scipe/biology/faculty/harnden/2122/images/esophagus.jpg
Presenter
Presentation Notes
-Fn: Coordinates food transit from the oral cavity to the stomach -Proximal boundary: Upper esophageal sphincter -Distal boundary: Lower esophageal sphincter -Contact time is typically less than 10 seconds, but the exposure may be significant because foods have not undergone any digestive processing -Normal esophagus contains a baseline number of T cells and dendritic cells that likely participate in health and disease states

Esophagus: Histology
http://faculty.une.edu/com/abell/histo/esophagusw.jpg

Esophagus: Histology
http://www.lab.anhb.uwa.edu.au/mb140/corepages/oral/images/oes041he.jpg

Esophagus: Histology
http://www.lab.anhb.uwa.edu.au/mb140/corepages/oral/images/oes041he.jpg
• Nonkeratinized squamous epithelium
• Protects the
body from swallowed materials and acid

Esophagus: Histology
http://www.lab.anhb.uwa.edu.au/mb140/corepages/oral/images/oes041he.jpg
• Submucosal glands • Secrete
bicarbonate and mucus

Esophagus: Histology
http://missinglink.ucsf.edu/lm/ids_101_histo_resource/images/222X10_copy.jpg
Presenter
Presentation Notes
Epithelium is composed of -stratum basale, which abuts the lamina propria and provides a constant renewal source of luminal epithelial cells -stratum intermedium -and the stratum superficialis -normal esophageal epithelium is usually devoid of eosinophils

Esophageal epithelium
• Intercellular attachment + control of permeability: – Desmosomes, adherens junctions, and tight
junctions
• Tight junction proteins: – Claudins, occludins, and junctional
adhesion molecules
• Adherens junction protein: – E-cadherin
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-Tight junction protein: Zonula occludins protein 1 (zo-1) perform the additional function of linking claudins and occludins to the actin cytoskeleton -Adherens junction protein: E-cadherin Surrounds cell membranes and supports adhesion of tight junction proteins -Functional loss of E-cadherin leads to decreased esophageal epithelial integrity and increased permeability

Esophageal epithelium
• Junctional proteins: – Superficial cell layers
• Claudins 1 and 4 • Occludin • Zo-1
– Intermediate and suprabasal layers • Claudins 1 and 4 • Occludin
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Esophagus: Histology
http://www.dartmouth.edu/~anatomy/Histo/lab_5/GI/DMS127/05.gif
Presenter
Presentation Notes
-Nonfibrotic, reticular network connecting the epithelium to the muscularis mucosa -LP contains T and B cells, eosinophils, and mast cells -Lymphoid aggregates exist within the LP of the esophagus, but their role in antigen presentation and tolerance is unclear

Esophagus: Histology
http://www.dartmouth.edu/~anatomy/Histo/lab_5/GI/DMS127/05.gif
Presenter
Presentation Notes
-muscularis mucosa: -Thickens from the proximal to the distal esophagus . -Muscularis propria: 2 layers -Internal: Concentric muscle fibers -External: Longitudinal muscle fibers -Actions of the proximal esophagus are coordinated by striated muscle -Actions of the mid- and distal esophagus are coordinated by smooth muscle

Stomach: Overview
http://www.paradoja7.com/wp-content/uploads/2013/12/anatomy-of-the-stomach.jpg
Presenter
Presentation Notes
-3 anatomically distinct sections—cardia, body (corpus, fundus), and antrum -stomach’s primary function is to prepare ingested food products for digestion and absorption -The stomach initially acts mechanically and biochemically to disrupt large pieces of food and serves as a reservoir for residual chyme, the semisolid mass of partially digested foodstuffs

Stomach: Cardia
http://www.paradoja7.com/wp-content/uploads/2013/12/anatomy-of-the-stomach.jpg
• Consists of a narrow strip of cells • Epithelia are primarily composed of
mucous cells • Protect the esophagus from gastric acidity

Stomach: Corpus
http://www.paradoja7.com/wp-content/uploads/2013/12/anatomy-of-the-stomach.jpg
• Breakdown of food: • Mechanical = Rugal folds • Biochemical = Acid and
pepsin • Layer of secreted
molecules: • Mucus, trefoil factors,
bicarbonate, acid, defensins, and prostaglandins
• Protects epithelial surfaces
Presenter
Presentation Notes
-Cellular interface is composed of columnar epithelia and a series of tightly packed tubular glands that contain acid-secreting parietal cells and pepsin-secreting chief cells -Underlying the epithelia are cells and molecules that form the structural and metabolic framework integral in supporting mucosal integrity -Resident cells include lymphocytes, endothelia, fibroblasts, myocytes, nerve cells, and a scattering of eosinophils -Absent of neutrophils

Stomach: Antrum
http://www.paradoja7.com/wp-content/uploads/2013/12/anatomy-of-the-stomach.jpg
• Antrum • Reservoir for
chyme • releases chyme
into the small intestine
Presenter
Presentation Notes
antrum contains a series of glands composed of mucous and endocrine cells

Stomach: Overview
http://media.web.britannica.com/eb-media/15/74315-036-756CBAC8.jpg

Stomach: Resident cells
http://cnx.org/content/m46517/latest/2415_Histology_of_StomachN.jpg
Presenter
Presentation Notes
-Parietal cells produce acid -Recepters controlling acid production: 1) Muscarinic (M3) receptors are stimulated by parasympathetic vagal nerve–derived acetylcholine 2) Cholecystokinin (CCKB) receptors are stimulated by gastrin that is released from duodenal G cells following exposure to protein. 3) gastrin and acetylcholine can stimulate production and release of histamine from enterochromaffin-like (ECL) cells -Stimulation of these receptors is activation of the hydrogen-ATPase pump, -> acid secretion and an intraluminal gastric pH of 2 -Antagonism of all 3 stimulatory receptors would be required to inhibit acid secretion completely -Prostaglandin E2 receptors are inhibitory to proton secretion. These receptors are stimulated by somatostatin that is produced by D cells located in the corpus, antral and small intestinal mucosa -Whether or not lack of acid contributes to allergic diseases has not been determined, but some evidence suggests that host sensitization to specific food proteins may be increased in the absence of acid

Stomach: Resident cells
http://cnx.org/content/m46517/latest/2415_Histology_of_StomachN.jpg
Presenter
Presentation Notes
-Pit cells: Secrete mucus that functions as a mechanical barrier, antimicrobial shield, and lubrication for foods -Mucus contains a number of structurally distinct glycoproteins, antimicrobial peptides, bicarbonate, and water -acetylcholine is the primary physiologic stimulant of mucus production -Secretin and prostaglandins also can also stimulate secretion

Stomach: Resident cells
http://cnx.org/content/m46517/latest/2415_Histology_of_StomachN.jpg
Presenter
Presentation Notes
-Primary function of chief cells: Pepsinogen synthesis and release -Production is response to stimulation by acetylcholine, histamine, or cholecystekinin

Small intestine: Overview
http://www.mhhe.com/biosci/ap/dynamichuman2/content/gifs/0124.gif
Presenter
Presentation Notes
-Largest portion of the GI tract. -Surface area, (folds, villi and microvilli) is approximately the size of a tennis court. -Length, ranging from 6-10 m -Boundary: the pylorus to ileocecal valve -Digestion and absorption are the most recognized functions -Whether deficiencies or excesses of these digestive processes are linked to allergic diatheses has not been determined -Other major function: Presentation and processing of Ag. It is uncertain how the system defines which antigens will adhere to the apical epithelial surface and undergo processing

Small intestine: Cells
http://media-2.web.britannica.com/eb-media/18/74318-004-2CEECF6F.jpg
Presenter
Presentation Notes
-Mucosal surfaces are composed of villi and crypts (crypts of Lieberkuhn) -Villous surfaces comprise absorptive columnar cells, Goblet cells, intraepithelial lymphocytes, and endocrine cells

Small intestine: Cells
http://medcell.med.yale.edu/systems_cell_biology_old/gi/images/stomach_small_intestine_cartoon.jpg
Presenter
Presentation Notes
-Villous surfaces comprise absorptive columnar cells, Goblet cells, intraepithelial lymphocytes, and endocrine cells -Crypts are also lined with stem cells, endocrine cells, and Paneth cells that serve to renew the villous epithelia and maintain epithelial health.

Small intestine: Cells
http://medcell.med.yale.edu/systems_cell_biology_old/gi/images/stomach_small_intestine_cartoon.jpg
Presenter
Presentation Notes
Overlying lymphoid follicles and PPs is a single layer of columnar cells termed the follicle-associated epithelium (FAE).

Small intestine: Cells
MT Abreu, Nature Reviews Immunology 2010, 131-143
Presenter
Presentation Notes
-Centrally located within the FAE are specialized microfold (M) cells. -M cells are different from absorptive epithelia they do not contain microvilli or membrane-associated hydrolytic enzymes and contain less glycocalyx. -M cells contain a characteristic feature, the invaginated subdomain within the basolateral membrane that forms an intraepithelial “pocket,” which is thought to function in antigen processing and presentation

Colon: Overview
http://www.crcftlauderdale.com/images/anat_colon_1.0.jpg
Presenter
Presentation Notes
-Boundary: ileocecal valve, to anal sphincter. -Length of 1.5 m in adults, divided into the cecum; ascending, transverse, descending, and sigmoid colon; and rectum. -Functions: Absorb water so that a formed stool can be expelled, and harbors the intestinal microbiome

Colon: Overview
http://www.as.miami.edu/chemistry/2086/Chap%2024/Chapter%2024-newPART2_files/image019.jpg
Presenter
Presentation Notes
-Absorptive columnar epithelial cells line mucosal surfaces and are interspersed by crypts that contain goblet, endocrine, stem cells, lymphocytes, endothelia, fibroblasts myocytes, endothelia, nerve cells, mast cells and a scattering of eosinophils. -Neutrophils are distinctly absent -A layer of secreted molecules that includes mucus, trefoil factors and defensins protect epithelial surfaces

GI IMMUNE SYSTEM

GI immune tissue
• Intraepithelial lymphocytes • Dense population of resident immune
cells in lamina propria • Organized lymphoid structures
– Payer’s patches (PP) – Isolated lymphoid follicles (ILF) – Mesenteric lymph nodes
• Further divided into effector and inductive sites
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Inductive sites
• Organized lymphoid structures consist of: – Naïve T cells, B cells, and APCs – Draining lymph nodes – Specialized lymphoid structures:
• PPs in the small intestine • Similar lymphoid tissues in the rectum • Isolated lymphoid follicles • Cryptopatches
• After activation in these organized tissues, Ag-specific T and B cells hone in on effector sites
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Inductive sites: Payer’s patch
CN Anderson, Nature Reviews Immunology 2001, 59-67
Presenter
Presentation Notes
-Peyer’s patch contains B-cell follicles. -The follicle-associated epithelium (FAE) covers the dome of the Peyer’s patch. -Transport across the epithelium occurs through both specialized M cells and by DCs that extend their processes through epithelial tight junctions. -DCs are present in both the subepithelial dome (SED) and the interfollicular T-cell areas and are visible as stellate (red) cells in these sites.

Effector sites: Lamina propria
• Phagocytes – Engulf and kill microbes
• Cytotoxic T cells – Kill infected cells
• B cells – Produce neutralizing antibodies
• Helper T cells – Production of cytokines
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Effector sites: Lamina propria
CN Anderson, Nature Reviews Immunology 2001, 59-67
Presenter
Presentation Notes
-The intestinal villus epithelium contains an unusual population of intraepithelial lymphocytes (IELs) which reside above the epithelial basement membrane. -Scattered lamina propria (LP) effector cells — T cells (T), IgA-secreting B cells (B) and DCs — are located within the villi

INNATE IMMUNITY IN GI TRACT

Components
• Mucosal barriers • Antimicrobial peptides • TLRs and NLRs • Intestinal microbiomes • Immune cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Mucosal barriers: Anatomy
• Extracellular components of the barrier – Epithelial cell surface – Hydrated gel formed by mucins – Unstirred layer of fluid
• Cellular components of the mucosal barrier – Epithelial cell membrane – Paracellular pathway: apical junctional complex
• Tight junction • Adherens junction
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Epithelial cell plasma membrane: impermeable to most hydrophilic solutes in the absence of specific transporters -Both tight and adherens junction are supported by a dense perijunctional ring of actin and myosin that, can regulate barrier function

Mucosal barriers: Anatomy
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Mucins are secreted by specialized epithelial cells, such as gastric foveolar mucous cells and intestinal goblet cells, and create a barrier that prevents large particles,including most bacteria, from directly contacting the epithelial cell layer -Defective mucus production has also been reported in various immunemediated diseases -Spontaneous colitis develops in mice that lack specific mucin genes -In the stomach, this property of the unstirred layer works with epithelial cell bicarbonate secretion to maintain a zone of relative alkalinity at the mucosal surface -The unstirred layer of the small intestine slows nutrient absorption by reducing the rate at which nutrients reach the transporting protein-rich microvillus brush border -May also contribute to absorption by limiting the extent to which small nutrients released by the activities of brush border digestive enzymes are lost by diffusion into the lumen -increased unstirred layer thickness has been reported in coeliac disease, in which it may contribute to nutrient malabsorption

Mucosal barriers: Anatomy
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Plasma membranes of adjacent cells seem to fuse at the tight junction, where claudins, zonula occludens 1 (ZO1), occludin and F‑actin interact. -E‑cadherin, α‑catenin 1, β‑catenin, catenin δ1 (also known as p120 catenin; not shown) and F‑actin interact to form the adherens junction -Myosin light chain kinase (MLCK) isassociated with the perijunctional actomyosin ring.

Mucosal barriers: Anatomy
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Desmosomes, which are located beneath the apical junctional complex, are formed by interactions between desmoglein, desmocollin, desmoplakin and keratin filaments

Mucosal barriers: Anatomy
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Adherens junctions and desmosomes, provide the strong adhesive bonds that maintain cellular proximity and are also a site of intercellular communication -Adherens junctions are composed of cadherins, a family of transmembrane proteins that form strong, homotypic interactions with molecules on adjacent cells. -The cytoplasmic tail of the epithelial cadherin, E-cadherin (also known as cadherin-1), interacts directly with catenin δ1(also known as p120 catenin) and β-catenin -In turn,β-catenin binds to α-catenin 1, which regulates local actin assembly and contributes to development of the perijunctional actomyosin ring -Adherens junctions are required for assembly of the tight junction

Mucosal barriers: Anatomy
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-Tight junctions are multi-protein complexes composed of transmembrane proteins, peripheral membrane (scaffolding) proteins and regulatory molecules that include kinases -The most important of the transmembrane proteins are members of the claudin family, which define several aspects of tight junction permeability -Claudins are expressed in a tissue-specific manner, and mutation or deletion of individual family members can have profound effects on organ function. -Occludin, a transmembrane tight junction protein that interacts directly with claudins and actin, is less well understood -Peripheral membrane proteins, such as zonula occludens 1 (Zo1) and Zo2, are crucial to tight junction assembly and maintenance -Tight junction is the rate-limiting step in transepithelial transport and the principal determinant of mucosal permeability -Specific barrier properties of the tight junction: size selectivity and charge selectivity

Transportation across tight juction
• At least 2 routes found – Leak pathway
• Allows paracellular transport of large solutes • Limited flux of proteins and bacterial
lipopolysaccharides • Size of particle exclusion has not been precisely
defined, but whole bacteria cannot pass • This pathway does not show charge selectivity • Flux across the leak pathway may be increased
by cytokines
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
Flux across the leak pathway may be increased by cytokines, including IFN-γ in vitro and TnF in vitro and in vivo

Transportation across tight juction
• At least 2 routes found – Small pores
• Defined by tight junction-associated claudin proteins
• Primary determinants of charge selectivity • Pores radius: 4 Å • Expression of specific claudins varies between
organs and within different regions of a single organ and can be modified by external stimuli, such as cytokines
• Properties may be regulated by physiological or pathophysiological stimuli
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-TnF-specific antibodies mainly reduce the inflammation, and corrects barrier dysfunction in patients with Crohn’s disease -Increased mucosal TnF production may also contribute to increased intestinal permeability and susceptibility to colitis in mice with defective -TnF-induced MLCK activation (MLCK=myosin light chain kinase) which alter the tight junction permeability and cause diarrhea mucin biosynthesis

Leak pathway regulatory measures
• MLCK activation – Clearly important
• Myosin ATPase – Its activity is regulated by MLC phosphorylation
• Members of the Rho kinase family – Phosphorylate MLC directly – Inhibit MLC phosphatase
• AMP-activated protein kinase – Activated during stress – Directly phosphorylate MLC
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
-MLCK=myosin light chain kinase -Rho kinases and AMP-activated protein kinase each have diverse effects that are separate from myosin function -Some of these contribute to tight junction regulation

Effect of cytokines on tight juction
• a | Occludin is normally concentrated at the tight junction (arrow) in jejunal villus epithelium. Perijunctional actomyosin ring :red, nuclei: blue
• b | Exogenous TNF increases myosin light chain kinase (MLCK) activity, which causes perijunctional myosin II regulatory light chain phosphorylation and triggers occludin (green) endocytosis (arrow). This increases flux across the tight junction leak pathway and enhances paracellular permeability to large solutes
JR Turner, Nature Reviews Immunology 2009, 799-809

Pore regulatory measures
• Via synthesis and trafficking of claudin proteins
• Expression of specific claudin proteins changes during: – Development – Differentiation – Disease – Response to stressors — including cytokines
• Claudin proteins associated with control of cell and organ growth
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
some changes in claudin protein expression enhance cell proliferation and regeneration, as might be necessary to compensate for cell loss in colitis

Pore regulatory measures
• Claudin-1 – Increased expression by intestinal epithelial cells
of IBD patients – Enhance neoplastic transformation, tumour
growth and metastasis in experimental models • Claudin-2
– Increased expression by intestinal epithelial cells in animal models of colitis and IBD patients
– IL-13 and IL-17 (increased in the mucosa of patients with colitis) increase claudin-2 expression in vitro, and reduce barrier function
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
it is not clear whether increased claudin-2 expression contributes to disease progression or is an adaptive response that promotes homeostasis

Effect of cytokines on tight juction
• c | Claudin-2 expression (green) is limited to crypt epithelial cells. F-actin: red, and nuclei: blue
• d | IL-13 can stimulate claudin-2 expression (green) in surface epithelial cells (arrows). This increases flux across small tight junction pores, enhancing paracellular cation permeability
JR Turner, Nature Reviews Immunology 2009, 799-809

Barrier defect related diseases
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
CCL2, CC‑chemokine ligand 2 DSS, dextran‑sulphate sodium EPEC, enteropathogenic Escherichia coli JAM‑A, junctional adhesion molecule‑A LPS, lipopolysaccharide MDR1A, multidrug resistance protein 1a MLCK, myosin light chain kinase NOD, nucleotide‑binding oligomerization domain TNBS, trinitrobenzene sulphonic acid ZO, zonula occludens

Barrier defect related diseases
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
CCL2, CC‑chemokine ligand 2 DSS, dextran‑sulphate sodium EPEC, enteropathogenic Escherichia coli JAM‑A, junctional adhesion molecule‑A LPS, lipopolysaccharide MDR1A, multidrug resistance protein 1a MLCK, myosin light chain kinase NOD, nucleotide‑binding oligomerization domain TNBS, trinitrobenzene sulphonic acid ZO, zonula occludens

Barrier defect alone is not enough
• Increased paracellular permeability: – Increase mucosal immune activity – Enhance disease progression and severity – Possible risk factor for development of
disease – But not enough to cause the disease!
• Restoration of tight junction barrier function may be effective in: – Preventing disease in at-risk individuals – Maintaining remission in IBD patients
JR Turner, Nature Reviews Immunology 2009, 799-809

Barrier loss & immune regulation
• Insufficient to cause disease
JR Turner, Nature Reviews Immunology 2009, 799-809
Tight junction barrier dysfunction alone

Barrier loss & immune regulation
• Insufficient to cause disease
JR Turner, Nature Reviews Immunology 2009, 799-809
Endoscopic mucosal resection
Presenter
Presentation Notes
Endoscopic mucosal resection removes the epithelium and mucosa completely and therefore causes barrier loss far greater than that caused by targeted tight junction dysfunction

Barrier loss & immune regulation
• Still…insufficient to cause disease
JR Turner, Nature Reviews Immunology 2009, 799-809
IBD + Endoscopic mucosal
resection
Presenter
Presentation Notes
So…robust immunoregulatory mechanisms must be induced by the host following barrier loss to prevent inappropriate inflammatory responses

Tight junction&immune regulation
JR Turner, Nature Reviews Immunology 2009, 799-809
Presenter
Presentation Notes
Minor barrier defects allow bacterial products and dietary antigens to cross the epithelium and enter the lamina propria -If the foreign materials are taken up by APCs, such as dendritic cells, that direct the differentiation of T helper 1 (TH1) or TH2 cells, disease can develop. -In this process, APCs and TH1 cells can release TNF and IFNγ, which signal to epithelial cells to increase flux across the tight junction leak pathway, thereby allowing further leakage of bacterial products and dietary antigens from the lumen into the lamina propria and amplifying the cycle of inflammation. This may, ultimately, culminate in established disease -Alternatively, IL‑13 released by TH2 cells increases flux across small cation‑selective pores, potentially contributing to ongoing disease. -Conversely, homeostasis may dominate if APCs promote Treg cell differentiation, which can be enhanced by epithelial cell‑derived TGFβ and retinoic acid. -TReg cells display latency‑associated peptide (LAP) on their surfaces and may secrete IL‑10 and TGFβ to prevent disease

Antimicrobial peptides
• Esophagus – Mucous layer of mucin-2 and glycoproteins – Trefoil factors (TFFs)
• Protease-resistant peptides • Produced by goblet cells • Secreted into the mucous layer • Promote epithelial cell survival and migration • Critical for immune defense and epithelial
barrier function
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
In multiple models of mucosal injury, recombinant TFF therapy can decrease the severity of epithelial injury and decrease the time to epithelial reconstitution

Antimicrobial peptides
• Small intestine – Mucous layer – Secretory immunoglobulin (sIgA) – Antimicrobial peptides – Mucins play an important role in normal
intestinal immune regulation
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
mucin-2 deficiency causes spontaneous inflammation and cancer in murine models

Antimicrobial peptides
• Defensins families – α-defensins:
• Human neutrophil peptides (HNP) 1 to 6 • Human α-defensin 5 and 6: From Paneth cell
– β-defensins: • Human β-defensins (HBD)
– HBD1: Multiple locations – HBD2: Small intestine and stomach – HBD3: Esophagus and oral cavity – HBD4: Gastric antrum
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-The α- and β-defensins and cathelicidins are normally expressed in the intestinal and colonic epithelial mucosa as well as in intestinal leukocytes -Difference in normal bacterial flora from mouth to anus is reflective of and maintained by the antibacterial spectrum of the antimicrobial peptides -Generally, β-defensins are expressed throughout the intestine by epithelial cells -HBD1: expressed in the epithelial cells of multiple gastrointestinal locations, including the small intestine and colon -HBD2: expressed only at low levels at baseline in the small intestine but can be upregulated in both the intestine and the stomach during inflammatory states

Antimicrobial peptides
• Cathelicidins (Human LL-37) • Cryptidins 1 to 6 • Phospholipase A2: Paneth cell • Lysozyme: Paneth cell
• Defensins and cathelicidins have
chemotactic properties for neutrophils, dendritic cells, and memory T cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
Cryptidin 4: against E. coli, not expressed in small intestine but is expressed at high levels in the colon

TLRs and NLRs: PRRs • Pattern recognition receptors (PRRs):
– Receptor molecules expressed in immune cells – Recognize pathogen-associated molecular patterns
(PAMPs) • Lipopolysaccharide • Flagellin • Bacterial DNA and RNA
– Have crucial roles in innate immunity and protection against pathogens
– Also recognize gut-resident microbiota • Most PRRs fall into 3 families:
– Toll-like receptors (TLRs) – NOD-like receptors (NLRs) – Retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs)
N Kamada, et al., Nature Reviews Immunology 2013, 321-335

Roles of TLRs in GI tract
• Sensing bacteria by the intestinal epithelium
• Sensing intestinal injury • Regulate barrier function
MT Abreu, Nature Reviews Immunology 2010, 131-143.

Expression of TLRs
• Localization of TLRs in GI mucosa – By anatomical location – By cell lineage – Spatial restriction by polarization – Intracellular TLRs: TLR4, found in-vitro
• Negative regulations of TLRs in GI mucosa
MT Abreu, Nature Reviews Immunology 2010, 131-143.

Anatomical location of TLRs
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
FAE, follicle associated epithelium IHC, immunohistochemistry M cell, microfold cell NEC, necrotizing entercolitis ND, not determined; NE, not expressed NF-κB, nuclear factor-κB TFF3, trefoil factor 3 WB, western blot ZO1, zonula occludens 1

Anatomical location of TLRs
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
FAE, follicle associated epithelium IHC, immunohistochemistry M cell, microfold cell NEC, necrotizing entercolitis ND, not determined; NE, not expressed NF-κB, nuclear factor-κB TFF3, trefoil factor 3 WB, western blot ZO1, zonula occludens 1

Polarization of TLRs
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
-In the human small intestine, the expression of TLR3, TLR4 and TLR5 has been shown on the basolateral surfaces of villus enterocytes -In the human colon, TLR3 and TLR5 are abundantly expressed, whereas TLR2 and TLR4 expression is low. -TLR9 has been shown to have contrasting effects on nuclear factor-κB (NF-κB) following apical or basolateral ligation

Negative regulations of TLRs
• Toll-interacting protein (ToLLIP) – An intracellular protein that inhibits TLR2 and
TLR4 signalling through its effect on IL-1R associated kinases (IRAKs)
– Expressed by IECs in vitro, especially following stimulation with LPS or lipotechoic acid
– LPS-induced inhibition of TLR activation = LPS tolerance
• IECs from IBD patients failed to upregulate Tollip expression – This may contribute to chronic inflammation
MT Abreu, Nature Reviews Immunology 2010, 131-143.

Negative regulations of TLRs
• Single immunoglobulin IL-1R-related molecule (SIGIRR; also known as TIR8) – Negative regulator of IL-1R, IL-33R, TLR4
and TLR9 signaling – Highly expressed by IECs – when deleted, made animals susceptible to
intestinal inflammation
MT Abreu, Nature Reviews Immunology 2010, 131-143.

TLRs and epithelial injuries
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
-Damage to intestinal epithelial barrier -> exposure of TLRs on the basolateral surface to bacterial products -TLR signalling in IECs might promote the proliferation of epithelial stem cells located at the base of colonic crypts by inducing the production of ligands for epidermal growth factor receptor (EGFR), such as amphiregulin (AR), and PGE2 by IECs �-PGE2 production can also be induced by TLR signalling in macrophages and COX2-expressing mesenchymal stromal cells (PSCs) -PSCs undergo repositioning to the stem cell niche in response to injury by which they can promote stem cell proliferation -ATF2, activating transcription factor 2; IκB, inhibitor of NF-κB

TLRs and epithelial injuries
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
-b | Recognition of LPS by TLR4 and its cofactors CD14 and MD2 triggers signalling through MYD88, IL-1R-associated kinases (IRAKs), TNFR-associated factor 6 (TRAF6) and TGFβ-activated kinase 1 (TAK1), resulting in the activation of NF-κB and mitogen-activated protein kinases (MAPKs) -In IECs, this leads to the release of EGFR ligands, such as AR, -> activate EGFR signalling -> proliferation -TLR4 signalling by IECs can also induce the expression of COX2 and the secretion of PGE2, which, through its receptors EP2 or EP4, can further stimulate the expression and release of AR by IECs -ATF2, activating transcription factor 2; IκB, inhibitor of NF-κB

TLRs and barrier functions
MT Abreu, Nature Reviews Immunology 2010, 131-143.
Presenter
Presentation Notes
-TLR stimulation of intestinal epithelial cells (IECs) -> expression of a proliferation-inducing ligand (APRIL) and thymic stromal lymphopoietin (TSLP), which are cytokines that promote class switch recombination (CSR) of IgM and IgA1 to proteaseresistant IgA2 -IgA2 binds bacteria at the apical surface of IECs to prevent bacterial invasion -Activation of TLR2 also stimulates the production of trefoil factor 3 (TFF3), which promotes the movement of cells necessary to repair gaps in the epithelial monolayer. -In response to TLR stimulation, Paneth cells secrete microbicidal peptides and lectins, such as α-defensins and regenerating islet-derived protein 3γ (REG3γ), respectively -PAMPs, pathogen-associated molecular patterns; pIgR, polymeric immunoglobulin receptor

NOD like receptors (NLRs)
• NOD=nucleotide-binding oligomerization domain-containing protein – NOD1 and NOD2
• Cytosolic proteins • Respond to intracellular fragments of bacterial
peptidoglycan -> NF-κB-dependent and MAPK-dependent gene transcription
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NOD like receptors (NLRs)
• NOD1 detects d-glutamylmeso-diaminopimelic acid (iE-DAP) – Dipeptide found in a peptidoglycan – Primarily found in Gram-neg bacteria but
also in select groups of Gram-pos bacteria, including Listeria spp. and Bacillus spp.
• NOD2 detects muramyl dipeptide (MDP) – Present in bacterial peptidoglycan
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NOD like receptors (NLRs)
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NODs expression
• NODs are expressed by – Epithelial cells – Stromal cells – Neutrophils – Dendritic cells – Paneth cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094. DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

Roles of NODs
• NLRP proteins interact with pyrin-containing proteins – Form inflammasomes – Cleave pro-IL-1β and pro-IL-18 into their
mature forms by using the caspase-1 pathway
• Activation of the caspase pathway -> pyroptosis
• NLRPs recognize both bacterial products (e.g., flagellin, toxins) and crystals (e.g., urea)
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
Pyroptosis = a form of cell death that causes cellular release of mediators that instigate and propagate inflammatory pathways.

NOD ligand recognition
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
-NOD1 and NOD2 can be recruited to the plasma membrane -NOD1 interacts with the tight junction-associated protein RHO guanine nucleotide exchange factor 2 (ARHGEF2), which facilitates the detection of invasive bacteria that exploit tight junctions for entry -NOD2 interacts with a complex of ARHGEF7 and ERBIN, as well as RAC1, which together mediate recruitment to the plasma membrane and pathogen-induced membrane ruffles -ATG16L1, autophagy-related protein 16‑like 1 -ERBIN, membrane-associated ERBB2‑interacting protein -FRMPD2, FERM and PDZ domain-containing protein 2

NOD ligand recognition
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
-NOD1 and NOD2 are able to recognize cytosolic fragments of peptidoglycan -Peptidoglycan fragments can be taken up by endocytosis and exported to the cytosol by solute carrier family 15 member 4 (SLC15A4) -Alternatively, gap junctions might be able to facilitate the movement of peptidoglycan fragments from infected cells to adjacent uninfected cells to drive NOD1 activation -The interaction of bacterial secretion systems with host membranes can also mediate peptidoglycan delivery to the cytosol -Finally, extracellular peptidoglycan can be directly transported into the cytosol by pH-sensing regulatory factor of peptide transporter 1 (PEPT1) -ATG16L1, autophagy-related protein 16‑like 1 -ERBIN, membrane-associated ERBB2‑interacting protein -FRMPD2, FERM and PDZ domain-containing protein 2

Roles of NODs
• NOD signaling in the stromal compartment is required for formation of intestinal lymphoid follicles
• Interactions between NODs and the microbiome are also essential
• NODs are also required for appropriate microflora control
• NOD2 deficiency increases bacterial load and decreases the clearance of bacteria from intestinal crypts
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

NOD signaling
• Nucleotide-binding function – NBDs of NOD1 and NOD2 mediate ligand-
induced oligomerization and hydrolyse ATP – ATP binding is required for NOD1 and NOD2
activation – ATP hydrolysis is required for deactivation
of NOD2 – Mutations that is important for ATP
hydrolysis associate with Blau syndrome and early-onset sarcoidosis (caused by auto-activation of NOD2)
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NOD signaling
• Activation and regulation of RIP2 – Sensing their respective peptidoglycan
ligands – NOD1 and NOD2 auto-oligomerization – Activation of RIP2 – Recruitment and activation of the TAK1–
TAB2–TAB3 complex – Drives IKK complex activation – IκBα phosphorylation and degradation
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
-TAK1 (TGFβ-activated kinase 1; also known as NR2C2) -TAB2 (TAK1‑binding protein 2) -IKK (IκB kinase) -NF-κB inhibitor-α (IκBα)

Consequences of NODs activation
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
-Stimulation of NOD1 and/or NOD2 leads to RIP2 activation -Activated RIP2 increased autophagosome formation by activating MAPK/ERK kinase kinase 4 (MEKK4)–p38 signalling and/or extracellular signal-regulated kinase 1 (ERK1) and ERK2 signalling, which upregulates basal levels of autophagy -Kinase function of RIP2 is required for the phosphorylation of the serine/threonine protein kinase ULK1 at Ser555, and for the deactivation of the protein phosphatase 2A (PP2A) complex that negatively regulates autophagy induction downstream of p38 activation -RIP2‑dependent ULK1 activation also restricts inflammasome activation by targeting reactive oxygen species (ROS)-producing mitochondria for degradation through autophagy -Interaction of ATG16L1 with the NOD complex negatively regulates inflammatory cytokine responses in a manner that is independent of downstream autophagosome formation -LC3, ubiquitin-like protein LC3

Consequences of NODs activation
• Activation of the MAPKs extracellular signal-regulated kinase 1 (ERK1), ERK2, JUNN-terminal kinase (JNK) and p38 – Lead to expression of pro-inflammatory
immune factors • TNF • IL-6, • CC-chemokine ligand 2 (CCL2) • Neutrophil chemoattractants • CXC-chemokine ligand 8 (CXCL8; or IL-8) • CXCL2 • Various antimicrobial factors, including defensins
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

Consequences of NODs activation
• Recruitment and priming innate immune cells
• Primes adaptive immune responses and • Key driver of TH2-type immunity • Activation of IFN regulatory factor 7 (IRF7)
– > Transcription of type I IFN genes – > Activation of the IFN-stimulated gene factor
3 (ISGF3) complex – > Initiate transcriptional program associated
with viral infection DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NOD signaling and intestinal berrier
• NOD2 has a key role in intestinal homeostasis by – Detecting peptidoglycan
released from the gut microbiota
– Driving a physiological inflammatory programme through the kinase RIP2, leading to NF-κB activation
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
NF‑κB activation -> Stimulating the production of antimicrobial peptides and mucin, Restrain the microbiota and maintain a physical distance between the microorganisms and the gut epithelial cell -An early T helper 17 (TH17) cell response enhances barrier protection by inducing the production of interleukin‑22 (IL‑22) and regenerating islet-derived protein IIIγ (REGIIIγ), and the CC‑chemokine ligand 2 (CCL2)-mediated recruitment of LY6Chi monocytes enhances barrier surveillance

NOD signaling and intestinal berrier
• In Crohn’s disease – Associated with NOD2
mutations – Some perturbation, which may
include antibiotics or an infection, alters the protective inflammatory programme
– > breakdown of intestinal barrier
– > Maybe dysbiosis • Compensatory immune
activation pathways then drives chronic inflammation
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
CCR2, CC-chemokine receptor 2

NODs and diseases
• Loss-of-function mutations of NOD2 – Affecting NOD2-mediated peptidoglycan
sensing – Associated with Crohn’s disease
• Gain-of-function mutations in the NBD of NOD2 correlate with – Autoinflammatory diseases – Blau syndrome – Early-onset sarcoidosis
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.

NODs and diseases
DJ Philpott, et al., Nature Reviews Immunology 2014, 9-23.
Presenter
Presentation Notes
AOM, azoxymethane; Apc, adenomatous polyposis coli CNS, central nervous system DSS, dextran sulphate sodium EAE, experimental autommune encephalitis MDP, muramyl dipeptide UPEC, uropathogenic Escherichia coli

Intestinal microbiomes
• What is “normal gut microbiota”? – Attempts to identify core microbiota – the
microbiota that is shared among (most of) humans – have not reached a consensus
– 3 types of the microbiota – enterotypes have been proposed
– A recent study with largest cohort to date, showed that the concept of enterotypes certainly cannot be applied on the microbiota of infants and young children
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16

Intestinal microbiomes
• 4 phyla predominate in the human large intestine: – Firmicutes – Bacteroidetes – Actinobacteria – Proteobacteria
• These symbionts and the mucosal epithelium have evolved in a mutually beneficial coexistence
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Intestinal microbiomes
• It is not clear what represents normal intestinal microbiota
• Microbiota composition of healthy subjects differs from that of patients suffering from a number of different diseases
• Different microbiota composition related to a disease = dysbiosis
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16

Intestinal microbiomes
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16
Presenter
Presentation Notes
Phylogenetic tree representing the diversity of the human gut microbiota, with indicated microbial markers of dysbiosis associated with colorectal cancer (CRC), Clostridium difficile associated diarrhoea (CDAD), inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS) and type 2 diabetes (T2D)

Roles of gut microbiomes
• Digest the “resistant carbohydrates” by fermentation – End products of carbohydrate
fermentations: • Gas • Short chain fatty acids (SCFAs – acetic, propionic
and butyric acid)
– SCFAs show anti-inflammatory properties
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16
Presenter
Presentation Notes
-Resistant carbohydrates = cellulose, hemicelluloses, inulins and resistant starch -

Roles of gut microbiomes
• Protein fermentation – Protein degradation mainly occurs in distal colon – Product: Beneficial SCFAs, and also other
potentially toxic metabolites – Bacteria are able to produce extracellular
proteases – IBD and IBS have been linked to an increased
proteolytic activity of intestinal contents – Extracellular microbial proteases are likely to play
a role in these diseases’ etiology
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16

Roles of gut microbiomes
• Deconjugation of bile acids – Primarily performed by intestinal Bacteroides
in mouse model – Mechanism for colonic epithelium protection
from genotoxic agents – Secondary bile acids had anti-inflammatory
effect, (most likely mediated through activation of TGR5 receptor)
– Microbiota-mediated metabolism of bile acids is important for the pathogenesis of IBD
MR Stojanovic, Best Practice & Research Clinical Gastroenterology 27 (2013) 5–16

Roles of gut microbiomes
• Mediate gut immune system development
N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Segmented filamentous bacteria (SFB) and other commensal microorganisms activate lamina propria dendritic cells (DCs) and macrophages to induce T helper 17 (TH17) cells and TH1 cells through the production of interleukin‑1β (IL‑1β), IL‑6 and IL‑23 in the case of TH17 cells, and possibly IL‑12 in the case of TH1 cells (although the role of IL-12 in TH1 development in vivo in the gut remains to be confirmed) -TH17 cells regulate the gut microbiota community in an IL‑22- and regenerating islet-derived protein 3γ (REGIIIγ)-dependent manner -Clostridium spp. clusters IV and XIVa, polysaccharide A (PSA)+ Bacteroides fragilis and other microbiota stimulate intestinal epithelial cells, T cells, and lamina propria DCs and macrophages to promote the development and/or the activation of forkhead box P3 (FOXP3)+ regulatory T (TReg) cells

Roles of gut microbiomes
• Mediate gut immune system development
N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-The microbiota stimulates intestinal epithelial cells and DCs to promote IgA-producing B cell and plasma cell differentiation in the lamina propria -TLR activation on intestinal epithelial cells induces the secretion of B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL), which promote the differentiation of IgA-producing plasma cells -Intestinal epithelial cells also produce thymic stromal lymphoprotein (TSLP) to promote BAFF and APRIL expression by DCs. -Various types of DCs, secrete BAFF, APRIL, nitric oxide (NO), retinoic acid and tumour necrosis factor (TNF) to facilitate the expression of activation-induced cytidine deaminase (AID) and IgA class-switching in B cells -Follicular DCs (FDCs) also induce the differentiation of IgA-producing plasma cells in Peyer’s patches and isolated lymphoid follicles. -IgA that is produced by lamina propria B cells is secreted into the intestinal lumen (SIgA), where it alters microbiota composition and function.

Roles of gut microbiomes
• Mediate gut immune system development
N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Innate lymphoid cells (ILCs) that express retinoic acid receptor-related orphan receptor-γt (RORγt) and produce IL-22 (termed ILC3s) regulate the gut microbiome through the induction of REGIIIγ in intestinal epithelial cells. -The microbiota positively regulates the production of IL‑22 by RORγt+ ILC3s via an unknown mechanism. -In addition, the microbiota induces IL‑25 secretion by endothelial cells, which acts on lamina propria DCs, and the IL‑25‑activated DC subset suppresses IL‑22 production by RORγt+ ILC3s. -CX3CR1, CX3C-chemokine receptor 1; SAA, serum amyloid A protein; TGFβ, transforming growth factor‑β

Roles of gut microbiomes
• Help the host fighting pathogens
N Kamada, et al., Nature Reviews Immunology 2013, 321-335

N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Direct competition -Resident microorganisms directly inhibit the colonization and/or the proliferation of incoming enteric pathogens. -Commensal microorganisms can outcompete pathogens for shared nutrients, such as carbohydrates, amino acids and organic acids. -In addition, commensal bacterial strains produce fucose, which inhibits virulence factor expression by pathogenic Escherichia coli. . -Enteric pathogens have evolved strategies to overcome competition by commensal bacteria -Some pathogens can directly kill their commensal competitors -Pathogen-induced inflammation, which leads to increased epithelial cell turnover, provides nutrients that selectively promote the growth of pathogens. -Pathogens can localize to epithelium-associated niches that are devoid of commensal bacteria and use nutrients near the epithelium to escape direct competition with resident microorganisms.

N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Indirect mechanisms of competition -Commensal bacteria catabolize polysaccharides to generate short-chain fatty acid (SCFAs), such as acetate, which enhances intestinal epithelial cell barrier function. -In addition, commensal microbiota promotes the production of mucus and the release of antimicrobial peptides such as regenerating islet-derived protein 3γ (REGIIIγ) from epithelial cells to limit pathogen colonization and proliferation. -Innate immune cells, such as intestinal resident macrophages, neutrophils and some ILC3s, as well as TH1 cells, TH17 cells and IgA-producing B cells and plasma cells, are also activated by the microbiota to limit pathogen colonization.

Roles of gut microbiomes
• Help the host fighting systemic infection – Role in systemic antibacterial immune
responses • Peptidoglycan molecules derived from the
microbiota are found in the periphery and can prime peripheral blood neutrophils to facilitate their bactericidal capacity via NOD1 signalling
• This enhances host defence against systemic infection with Streptococcus pneumoniae
N Kamada, et al., Nature Reviews Immunology 2013, 321-335

Roles of gut microbiomes
• Help the host fighting systemin infection – Role in systemic antiviral immune responses
• Microbiota promotes type I IFN production by macrophages and IFN-priming of NK cells
– Role in systemic antiparasitic immune responses
• Microbiota induces intestinal TLR-dependent DC activation
– Microbiota might provide local tissue-specific defense mechanisms
N Kamada, et al., Nature Reviews Immunology 2013, 321-335

Roles of gut microbiomes • Roles on IBD
– Beneficial subsets of commensal bacteria: Anti-inflammatory activities
– Pathobionts are directly suppressed by beneficial commensal bacteria partly through the induction of regulatory immune responses, involving TReg cells, IL-10 and regenerating islet-derived protein 3γ (REGIIIγ)
N Kamada, et al., Nature Reviews Immunology 2013, 321-335

Roles of gut microbiomes
• Roles on IBD
N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Combination of genetic factors (for example, mutations in Nod2, autophagy-related gene 16‑like 1 (Atg16l1) and IL‑23 receptor (Il23r)) and environmental factors (such as infection, stress and diet) result in disruption of the microbial community structure, a process termed dysbiosis -Dysbiosis results in a loss of protective bacteria and/or in the accumulation of pathobionts, which leads to chronic inflammation involving hyperactivation of TH1 and TH17 cells -Dashed line shows that that the suppression of pathobionts by beneficial bacteria is diminished -In certain contexts, pathobionts can be transferred to the host and can cause disease without the host having a predisposing genetic susceptibility -It is unknown whether unclassified bacteria have a role in the pathogenesis of IBD. GALT, gut-associated lymphoid tissue

Other roles of microbiomes • Roles on IBD
N Kamada, et al., Nature Reviews Immunology 2013, 321-335
Presenter
Presentation Notes
-Segmented filamentous bacteria (SFB) colonization induces TH17 cell development in the intestine, which might migrate to the periphery to affect systemic and CNS immunity; increased intestinal TH17 cells enhance the expansion of pathogenic autoAg-specific T cells in the intestine and cause inflammation in CNS -By contrast, ‘beneficial’ commensal bacteria can attenuate CNS inflammation through the induction of FOXP3+ Treg Cells -Induced TH17 cells can also promote autoimmune arthritis by facilitating autoAb production by B cells -In addition, microbiota-induced IL‑1β signalling participates in the development of rheumatoid arthritis through the induction of TH17 cells. The IL‑1 receptor (IL‑1R) antagonist blocks IL‑1β signalling and abrogates joint inflammation -Balance in the microbial community also determines susceptibility to T1DM. A decreased Firmicutes/Bacteroidetes ratio as a result of a deficiency in MYD88 in non-obese diabetic mice is associated with an attenuated risk of T1DM. -SFB-induced TH17 cells protect the host against T1DM development by an unknown mechanism. -Exposure to microorganisms in neonatal, but not adult, life decreases the accumulation of invariant natural killer T (iNKT) cells in the gut, which results in protection against allergic inflammation in the lungs. In addition, microbial compounds stimulate peripheral B cells through B cell-intrinsic MYD88 signalling and inhibit IgE production. Decreased levels of peripheral IgE result in decreased numbers of basophils, and attenuate the risk of allergic airway inflammation. -EAE, experimental autoimmune encephalomyelitis

Immune cells
• Eosinophils • Mast cells • Innate lymphoid cells (ILC) • Multifunctional IgA+ plasma cells • Macrophage • Denritic cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Eosinophils
• Various location distribution – Normal esophageal epithelium: None – Lamina propria (LP) and muscularis mucosa
: have eosinophils – Lower GI tract has significant numbers of
eosinophils – eosinophil numbers are highest in the
cecum and rectosigmoid – Rarely found in the surface and crypt
epithelium
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
Presence of Eo in these locations may indicate a pathologic process

Eosinophils
• Intestinal accumulation is independent of bacterial colonization and presence of lymphocytes
• Function in nondiseased GI tract: Unknown – Recruited to sites of continual cell turnover ? – Regulate local immunity and tissue
repair/remodeling? – Parasite expulsion on chronic or repeat
infection can be dysregulated in the absence of eosinophils and IL-5
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Mast cells
• GI: 1 of the largest reservoirs • Esophageal and colonic epithelium is
normally entirely devoid of tryptase-positive cells
• Tryptase-positive cells are found in varying numbers through the colonic LP
• Tryptase-chymase double-positive mast cells predominate in the esophageal LP
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Mast cells
• GI mast cells are induced during immune responses to parasites
• Their normal function in GI immune homeostasis remains unclear
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Innate lymphoid cells
• Members: – Natural killer (NK) – Lymphoid tissue inducer (LTi) cells
• Required for lymph node formation during embryogenesis
• Produce IL-17 and IL-22
– Natural helper cells – Innate helper type 2 cells – Nuocytes
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-All of these cells produce the Th2 cytokines IL-5 and IL-13 -These cells do not express lineage markers found on B, T, NK or NKT cells, mast cells, basophils, granulocytes, dendritic cells, or macrophages

Innate lymphoid cells
• Relationship between these cell types is currently unclear
• Rapid and robust production of Th2 cytokines in response to IL-25 and IL-33 are common
• Through production of IL-5, innate lymphoid cells recruit eosinophils and are important for antihelminth immunity
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

IgA+ plasma cells
• They have B cell and monocytic and dendritic cell markers.
• Some of them produce the TNF-α and inducible nitric oxide synthase – Both of which are required to maintain
homeostasis of gut microbiota.
• The acquisition of these cells depends on microbial stimulation and gut stroma
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
which indicates a symbiotic relationship between the microbiome and the gut immune system

Macrophage
• GI mucosa: largest reservoir of mononuclear phagocytes in the body
• Intestinal macrophages are derived from circulating monocytes
• Resident macrophages are highly phagocytic
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Macrophage
• They can ingest microbes and function as scavengers without generating an inflammatory response
• Innate signaling molecules such as MyD88 and TRIF adapter proteins are decreased or absent in intestinal macrophages – Which explains the broad TLR
nonresponsiveness despite TLR expression
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Macrophage
• Functions: – Regulate the inflammatory response to the
normal flora – Respond to pathogens – Scavenge debris and dead cells – Phagocytosis and microbial killing
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Denritic cells
• Lamina propria contains a dense network of dendritic cells
• Functions: – Sample antigen – Initiation of adaptive immune responses
• Subset of lamina propria DCs expressing the markers CD11c and CD103 – Express high levels of the flagellin sensor TLR5 – But low levels of TLR4 in comparison to splenic
DCs
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-TLR5 is necessary for detection of the pathogen Salmonella typhimurium.

Denritic cells activation
• Activation through TLR5 leads to: – DC production of IL-23 – ILC expression of IL-17 and IL-22. – These cytokines are important for
antimicrobial defense in the intestine
• Human intestinal DCs are responsive to TLR3, TLR7, and TLR9 stimulation – May play important role in antiviral immunity
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-TLR5 is necessary for detection of the pathogen Salmonella typhimurium.

ADAPTIVE IMMUNITY IN GI TRACT

Components
• Antigen uptake • Antigen presentation • Food antigen: Immune tolerance • Microbial antigen: Immune exclusion
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Antigen uptake
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
Ag can cross the epithelial surface by at least three routes: A, passage through the M cells that overlie Peyer patches B, transcellularly; C, paracellularly through epithelia to underlying T cells or macrophages; and capture by dendritic cell (DC) processes that extend between epithelial cells

Antigen uptake
• Soluble Ag, including many food antigens, are taken up primarily across enterocytes lining the intestinal villus
• Under normal conditions, uptake of intact macromolecules occurs by a transcellular transport mechanism for depositing antigenic material across the basolateral surface of the epithelium
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Antigen uptake
• When exposed to an Ag, uptake of that Ag can be modified by the presence of Ig – IgA primarily results in immune exclusion – IgG and IgE can enhance uptake through
epithelial expression of immunoglobulin receptors (FcRn and CD23, respectively)
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Antigen presentation
• Antigen-presenting cells (APCs) in the GI tract – Professional APCs
• dendritic cells (DCs) • B cells • Macrophages
– Nonprofessional APCs • Epithelial cells.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Dendritic cells subsets
• At oral and esophageal mucosa – Subsets of DCs are distinct from lower GI tract – Greater similarity to DCs in the skin
• Langerhans cells • Interstitial DCs • Langerin+ interstitial DCs
• Oral DCs acquire and present Ag to T cells – They can generate either tolerance or protective
immunity, depending on the context of antigen administration
• Whether esophageal DCs can acquire and present ingested food Ag in vivo is unknown
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Dendritic cells subsets
• In small and large intestine: 2 DC subsets – CD11b+ CD103− DC
• Expresses the chemokine receptor CX3CR1 • Extends dendrites into the small intestinal lumen • Highly phagocytic • Efficiently capture Ag • Not migrate to draining mesenteric lymph node
(MLN) • Likely to play a role in recall responses rather than
in initiation of immune responses
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Dendritic cells subsets
• In small and large intestine: 2 DC subsets – CD11c+ CD103+ DC
• Low level of Ag capture • More efficient at Ag presentation • Transport Ag to the MLN for presentation to
naïve T cells • Initiation of immune responses to soluble Ag
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Dendritic cells subsets
• In the PPs – Subepithelial DCs migrate to T cell areas on
appropriate stimulation
– Some DC subsets are in the PPs • They can be differentiated by their surface
markers, eg. CD11b, CD8α, and CCR6.
• There is evidence the different DC phenotypes are specialized to respond to specific inflammatory stimuli or microbial challenges.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Mononuclear phagocytes
• CX3CR1+ phagocytes – No migratory activity – Process and present acquired Ag – Interact with resident T cells of the LP – Participate in the reactivation of memory T
cells – Local activation of effector T cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Mononuclear phagocytes
• CD11c− macrophages of the mouth and small intestines – Present Ag to T cells – Preferentially induce the development of
Tregs through an IL-10– and retinoic acid–dependent mechanism.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Epithelial cells
• Capable of presenting Ag to T cells • Induce the expansion of CD8+ T cells
with regulatory activity through a CD1d-dependent mechanism
• During inflammatory states, epithelial cells of the esophagus and small intestine upregulate MHC II and can activate CD4+ T cells
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Food Ag: Immune tolerance
• Intact Ag can be detected in the blood after a meal in healthy volunteers
• These Ag were ignored by immune system?:… NO! – Presence of food-specific IgG and IgA
antibodies in the serum of healthy individuals
• But no immune activation?
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Food Ag: Immune tolerance
• Oral tolerance – Oral administration of Ag resulted in the
generation of CD4+ and CD8+ T cells with regulatory or suppressive activity
• Transfer of CD4+ or CD8+ T cells from fed mice to naïve mice could transfer tolerance in an Ag-specific manner
• CD8+ T cells are not required for oral tolerance, but they are capable of mediating tolerance
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Food Ag: Immune tolerance
• Deletion of peripherally induced FOXP3+ CD4+ CD25+ Tregs can reverse oral tolerance
• DCs migrating from the small intestinal LP are uniquely specialized to program responder T cells to develop into Tregs.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
forkhead box P3 protein (FOXP3+)

Oral tolerance
MC Berin, et al., Current Biology 23, R389–R400, May 6, 2013
Presenter
Presentation Notes
-Ag are captured in the lamina propria and Peyer’s patch and carried to the mesenteric lymph node (MLN) by CD103+ DC, which induce gut-homing (a4b7+) induced regulatory T cells (iTregs) by a mechanism dependent on TGF-b, retinoic acid (RA) and indoleamine-2,3-dioxygenase (IDO). -DC induce gut-homing IgA-secreting plasma cells also through RA-dependent mechanisms. -Gut-homing iTregs are expanded in the lamina propria by IL-10-expressing CX3CR1+ macrophages. -These iTregs can then suppress systemic immune responses, including allergic sensitization, in an Ag-specific manner. -The immune mechanism of Th3 induction has not been established, but Th3 cells induced by feeding also suppress systemic immune responses

Food Ag: Immune tolerance
• Liver can also participate in the induction of oral tolerance – Mediated through plasmacytoid DCs – Induce the deletion of Ag-specific CD8+ T
cells. • Role of the MLN vs the liver in induction
of tolerance may depend on: – Nature of the Ag – How it is handled after breaching the
intestinal epithelial barrier
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Food Ag: Immune tolerance
• Immune tolerance can be induced through – Airways – Sublingual route
• Multiple sites along the GI tract likely contribute to the development of clinical tolerance to food antigens.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
box P3 protein (FOXP3+)

Microbial Ag: Immune exclusion
• GI tract: hyporesponsive to signaling through the TLRs
• Innate and adaptive immune system work in a coordinated manner to keep the flora compartmentalized to the GI mucosa
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Microbial Ag: Immune exclusion
• Absence of signaling through MyD88 and TRIF: – Compensatory systemic Ab response
against the intestinal flora – Protects against systemic dissemination of
bacteria
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
MyD88 and TRIF, the downstream signaling molecules of the TLRs,

Microbial Ag: Immune exclusion
• Commensal bacteria are normally sampled by the mucosal immune system and carried by migratory DCs to the draining MLN – They induce an IgA response partially
dependent on T cell help
• T cell–independent IgA class switching has also been documented in the intestinal LP.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Microbial Ag: Immune exclusion
• IgA induced by the flora is secreted into the intestinal lumen by epithelial cells expressing the polymeric immunoglobulin receptor (pIgR).
• But…IgA deficiency is associated with a relatively mild phenotype.
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
This may be caused by the overlapping functions of the innate and acquired immune systems in containing the intestinal flora

Microbial Ag: Immune exclusion
• The generation of a commensal flora–triggered IL-17 and IFN-γ response from T cells is associated with pathology in IBD
• Conflicting data on the role of the commensal flora in the development of intestinal Tregs
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

Microbial Ag: Immune exclusion
• In the presence of normal innate and adaptive immune responses within the GI mucosal immune system – Systemic immune system is kept ignorant of
the commensal flora – Contrast to the normal immune response to
food Ag, in which active immune tolerance is observed systemically
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.

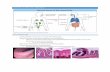
Conclusion
MC Berin, et al., Gastrointestinal Mucosal Immunology, Middleton’s Allergy 8th edition, 2013, 1084-1094.
Presenter
Presentation Notes
-The epithelial surface is exposed to an abundant and diverse microbiome. -To protect itself, it is coated with mucus that contains trefoil proteins, secretory IgA, and antimicrobial peptides. -Ag may pass through the epithelial surface and undergo processing in Peyer patches. -Within the subepithelial dome (SED) reside a number of lymphocytes, including natural helper T (Th0) cells, which can differentiate into type 1 (Th1), type 2 (Th2), regulatory (Treg), or Th17 cells. -Other resident cells include macrophages, cytotoxic lymphocytes, mast cells (MC), eosinophils (EOS), and dendritic cells (DC). -IEL, Intraepithelial lymphocytes

THANK YOU
Related Documents