Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study _ Ilhami Gu ¨lc ¸in 1,2 1 Department of Chemistry, Faculty of Sciences, Atatu ¨ rk University, Erzurum, Turkey. 2 School of Health Services, _ Ibrahim Cecen University, Agri, Turkey. ABSTRACT Eugenol (4-allyl-2-methoxyphenol), a major phenolic component from clove oil (Eugenia caryophyllata), has several biological activities. To estimate the capacity of eugenol to act as an antioxidant, the following were studied: 1,1- diphenyl-2-picryl-hydrazyl–, 2,2 0 -azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)–, and N,N-dimethyl-p-phenylenediamine– scavenging activity; total antioxidant activity; and ability to reduce ferric ions and cupric ions. Eugenol inhibited 96.7% (r 2 = 0.9319) lipid peroxidation of a linoleic acid emulsion at a 15-lg/mL concentration. Butylated hydroxyanisole, butylated hydroxytoluene, a-tocopherol, and Trolox Ò displayed 95.4% (r 2 = 0.8482), 99.7% (r 2 = 0.7798), 84.6% (r 2 = 0.9272), and 95.6% (r 2 = 0.8511) inhibition of peroxidation, respectively, at the 15-lg/mL concentration. According to the results of this study, eugenol had the most powerful antioxidant activity and radical-scavenging activity. This study should prompt further studies of the antioxidant properties of eugenol. KEY WORDS: antioxidant activity eugenol radical scavenging reducing power INTRODUCTION E ugenol (4-allyl-2-methoxyphenol), a methox- yphenol with a short hydrocarbon chain, is the major component (80%–95%) of clove oil. 1 Eugenol has been used as a spice because of its strong odor and as a dental antiseptic because of its detergent-like effect. 2,3 It has been extensively used as a therapeutic agent in dentistry for sedation in pa- tients with toothache, pulpitis, and dental hyperalgesia. 4,5 Pharmacologic studies have demonstrated that eugenol has anticonvulsant, 6 local anesthetic 7 and antistress, 8 bacterio- static and bactericidal, 9 anticandidal, 10 and antifungal 11 properties. In addition, eugenol has antigenotoxic 12 and anticarcinogenic 13 potential. Eugenol has induced reactive oxygen species–mediated apoptosis in HL-60 human pro- myelocytic leukemia cells. It depresses neuromuscular transmission 14 and central nervous system function. 6 Eu- genol prevented radiation-induced chemical oxidative damage in membranes and modified the membrane-associ- ated signaling process after radiation exposure. 15 In view of its nonmutagenic and noncarcinogenic prop- erties, eugenol is generally regarded as safe by the Food and Agricultural Organization of the United Nations, with an acceptable daily intake of up to 2.5 mg/kg body weight in humans. 16 Reactive oxygen species (ROS), such as superoxide, hy- drogen peroxide, and singlet oxygen, and other free radicals, such as nitrogen free radicals, appear to be generated in the human body because of various internal or external sources. ROS and other free radicals are byproducts of normal cel- lular metabolism in aerobic life, where molecular oxygen is ubiquitous. ROS are generated during irradiation by ultraviolet light, x-rays, and gamma rays; are products of metal-catalyzed reactions; are present as pollutants in the atmosphere; are produced by neutrophils and macrophages during inflammation; and are byproducts of mitochondria- catalyzed electron transport reactions and other mecha- nisms. 17 ROS may contribute to oxidative damage of lipids, protein, nucleic acids, and polyunsaturated fatty acids in living cells. Free radicals or ROS play important roles in the development of many chronic diseases, such as the aging process, heart disease, and cancer. 18 It is significant for health to look for efficient ways to decrease or depress creation of free radicals in the body. Oxidative stress has been defined as an imbalance be- tween the production of ROS and antioxidant defense. Be- cause of the disturbance in the equilibrium state of prooxidant-antioxidant reaction, ROS are overproduced to induce oxidative stress, which inhibits normal functions of cellular lipids, proteins, DNA, and RNA. Thus, increasing attention has been directed toward finding some natural antioxidant compounds that could be isolated from herbal medicine and efficiently clear free radicals. Antioxidants are a defensive factor against free radicals’ effects in the body. The antioxidants not only eliminate ROS but also adjust the cellular redox state and enable redox signal transduction. They act by inhibiting the initiation and propagation steps, leading to the termination of the reaction and delaying the Manuscript received 30 July 2010. Revision accepted 7 October 2010. Address correspondence to: _ Ilhami Gu¨lc ¸in, Atatu¨rk University, Faculty of Sciences, Department of Chemistry, TR-25240-Erzurum, Turkey, E-mail: [email protected] JOURNAL OF MEDICINAL FOOD J Med Food 14 (9) 2011, 975–985 # Mary Ann Liebert, Inc. and Korean Society of Food Science and Nutrition DOI: 10.1089/jmf.2010.0197 975

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study
_Ilhami Gulcin1,2
1Department of Chemistry, Faculty of Sciences, Ataturk University, Erzurum, Turkey.2School of Health Services, _Ibrahim Cecen University, Agri, Turkey.
ABSTRACT Eugenol (4-allyl-2-methoxyphenol), a major phenolic component from clove oil (Eugenia caryophyllata), has
several biological activities. To estimate the capacity of eugenol to act as an antioxidant, the following were studied: 1,1-
diphenyl-2-picryl-hydrazyl–, 2,20-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)–, and N,N-dimethyl-p-phenylenediamine–
scavenging activity; total antioxidant activity; and ability to reduce ferric ions and cupric ions. Eugenol inhibited 96.7%
(r2 = 0.9319) lipid peroxidation of a linoleic acid emulsion at a 15-lg/mL concentration. Butylated hydroxyanisole, butylated
hydroxytoluene, a-tocopherol, and Trolox� displayed 95.4% (r2 = 0.8482), 99.7% (r2 = 0.7798), 84.6% (r2 = 0.9272), and
95.6% (r2 = 0.8511) inhibition of peroxidation, respectively, at the 15-lg/mL concentration. According to the results of this
study, eugenol had the most powerful antioxidant activity and radical-scavenging activity. This study should prompt further
studies of the antioxidant properties of eugenol.
KEY WORDS: � antioxidant activity � eugenol � radical scavenging � reducing power
INTRODUCTION
Eugenol (4-allyl-2-methoxyphenol), a methox-
yphenol with a short hydrocarbon chain, is the majorcomponent (80%–95%) of clove oil.1 Eugenol has been usedas a spice because of its strong odor and as a dental antisepticbecause of its detergent-like effect.2,3 It has been extensivelyused as a therapeutic agent in dentistry for sedation in pa-tients with toothache, pulpitis, and dental hyperalgesia.4,5
Pharmacologic studies have demonstrated that eugenol hasanticonvulsant,6 local anesthetic7 and antistress,8 bacterio-static and bactericidal,9 anticandidal,10 and antifungal11
properties. In addition, eugenol has antigenotoxic12 andanticarcinogenic13 potential. Eugenol has induced reactiveoxygen species–mediated apoptosis in HL-60 human pro-myelocytic leukemia cells. It depresses neuromusculartransmission14 and central nervous system function.6 Eu-genol prevented radiation-induced chemical oxidativedamage in membranes and modified the membrane-associ-ated signaling process after radiation exposure.15
In view of its nonmutagenic and noncarcinogenic prop-erties, eugenol is generally regarded as safe by the Food andAgricultural Organization of the United Nations, with anacceptable daily intake of up to 2.5 mg/kg body weight inhumans.16
Reactive oxygen species (ROS), such as superoxide, hy-drogen peroxide, and singlet oxygen, and other free radicals,
such as nitrogen free radicals, appear to be generated in thehuman body because of various internal or external sources.ROS and other free radicals are byproducts of normal cel-lular metabolism in aerobic life, where molecular oxygenis ubiquitous. ROS are generated during irradiation byultraviolet light, x-rays, and gamma rays; are products ofmetal-catalyzed reactions; are present as pollutants in theatmosphere; are produced by neutrophils and macrophagesduring inflammation; and are byproducts of mitochondria-catalyzed electron transport reactions and other mecha-nisms.17 ROS may contribute to oxidative damage of lipids,protein, nucleic acids, and polyunsaturated fatty acids inliving cells. Free radicals or ROS play important roles in thedevelopment of many chronic diseases, such as the agingprocess, heart disease, and cancer.18 It is significant forhealth to look for efficient ways to decrease or depresscreation of free radicals in the body.
Oxidative stress has been defined as an imbalance be-tween the production of ROS and antioxidant defense. Be-cause of the disturbance in the equilibrium state ofprooxidant-antioxidant reaction, ROS are overproduced toinduce oxidative stress, which inhibits normal functions ofcellular lipids, proteins, DNA, and RNA. Thus, increasingattention has been directed toward finding some naturalantioxidant compounds that could be isolated from herbalmedicine and efficiently clear free radicals. Antioxidants area defensive factor against free radicals’ effects in the body.The antioxidants not only eliminate ROS but also adjust thecellular redox state and enable redox signal transduction.They act by inhibiting the initiation and propagation steps,leading to the termination of the reaction and delaying the
Manuscript received 30 July 2010. Revision accepted 7 October 2010.
Address correspondence to: _Ilhami Gulcin, Ataturk University, Faculty of Sciences,Department of Chemistry, TR-25240-Erzurum, Turkey, E-mail: [email protected]
JOURNAL OF MEDICINAL FOODJ Med Food 14 (9) 2011, 975–985# Mary Ann Liebert, Inc. and Korean Society of Food Science and NutritionDOI: 10.1089/jmf.2010.0197
975

oxidation process. The mechanism of antioxidants may in-volve the scavenging of free radicals.19,20
At present, a variety of synthetic antioxidants are com-monly used. However, the use of these compounds has beenrestricted by legislation because of doubts over their toxicand carcinogenic effects. Plant foods are potential sources ofnatural antioxidants, such as vitamin C, tocopherol, carot-enoids, flavonoid, and phenolic acids, that prevent freeradical damage. They can provide phenolic hydroxyl groupsto react with free radicals. Consequently, they will inhibitthe oxidative mechanisms that degenerative diseases.Hence, there is a growing interest in natural and safer an-tioxidants for food and pharmaceutical applications and agrowing trend in consumer preferences toward natural an-tioxidants. All of this has given impetus to attempts to ex-plore natural sources of antioxidants.21–23
This study investigated the following capacities ofeugenol: inhibition of lipid peroxidation in the linoleic acidsystem, ferric ion (Fe3+)–reducing antioxidant power(Fe3+–Fe3+ transformation), cupric ion (Cu2+)–reducingantioxidant power (CUPRAC method); 1,1-diphenyl-2-picryl-hydrazyl (DPPH�)–scavenging, 2,20-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS�+)–scavenging,N,N-dimethyl-p-phenylenediamine (DMPD�+)–scavenging,superoxide anion radical–scavenging, and hydrogen perox-ide (H2O2)–scavenging; and ferrous ion (Fe2+)–chelatingactivities of eugenol. These multiple methods are recom-mended to measure antioxidant properties of food orpharmacologic materials that better reflect their potentialprotective effects.
MATERIAL AND METHODS
Chemicals
The following compounds used for antioxidant activitieswere obtained from Sigma (Sigma-Aldrich GmbH): eugenol(4-allyl-2-methoxyphenol), neocuproine (2,9-dimethyl-1,10-phenanthroline), DMPD, ABTS, butylated hydro-xyanisole (BHA), butylated hydroxytoluene (BHT),DPPH�, 3-(2-pyridyl)-5,6-bis (4-phenyl-sulfonic acid)-1,2,4-triazine (Ferrozine�), linoleic acid, a-tocopherol,polyoxyethylenesorbitan monolaurate (Tween-20�), andtrichloroacetic acid. Ammonium thiocyanate was purchasedfrom Merck. All other chemicals used were of analyticalgrade and obtained from Sigma-Aldrich or Merck.
The following experimental procedures were applied toevaluate the performance of the eugenol and to establish, ifpossible, structure–activity relationships.
Determination of total antioxidant activity
The ferric thiocyanate method was used to evaluate theeffect of eugenol on the prevention of peroxidation of linoleicacid emulsion, as described elsewhere.21,24 A stock solutioncontaining 10 mg eugenol was dissolved in 10 mL ethanol.Eugenol (15 lg/mL) was prepared by diluting the stock so-lution in 2.5 mL sodium phosphate buffer (0.04 M; pH, 7.0),and these were added to 2.5 mL linoleic acid emulsion in
sodium phosphate buffer (0.04 M; pH, 7.0). The linoleic acidemulsion was prepared by homogenizing 15.5 lL linoleicacid, 17.5 mg Tween-20 as emulsifier, and 5 mL phosphatebuffer (pH, 7.0). The control was composed of 2.5 mL lino-leic acid emulsion and 2.5 mL 0.04 M sodium phosphatebuffer (pH, 7.0). The reaction mixtures (5 mL) were incu-bated at 37�C in polyethylene flasks. The peroxide levelswere determined by reading the absorbance at 500 nm in aspectrophotometer (UV-1208 UV-VIS Spectrophotometer;Shimadzu) after reactions with FeCl2 and thiocyanate at in-tervals during incubation.25–27 The peroxides formed duringlinoleic acid peroxidation oxidize Fe2+ to Fe3+, and Fe3+
forms a complex with thiocyanate that has a maximum ab-sorbance at 500 nm. The assay steps were repeated every 5hours until the maximum was reached. The percentage ofinhibition was calculated at this point (55 hours). The solutionwithout eugenol was used as the blank sample. Linoleic acidmixture without the addition of sample was used as control.The percentage of inhibition of lipid peroxidation in linoleicacid emulsion was calculated by the following equation:
Inhibition of lipid peroxidation (%)¼ 1�A500� S
A500�C
� �· 100
where A500-C is the absorbance of the control reaction,which contains only linoleic acid emulsion and sodiumphosphate buffer and A500-S is the absorbance of sample inthe presence of eugenol or other test compounds.21,28
Fe3+-Fe2+–reducing assay
Ferric-reducing antioxidant power was measured by thedirect reduction of Fe3+(CN-)6 to Fe2+(CN-)6 and was de-termined by measuring absorbance resulting from the for-mation of the Perl’s Prussian blue complex after the additionof excess ferric ions (Fe3+). Thus, the ferric-reducing anti-oxidant power method of Oyaizu29 with slight modificationswas used to measure the reducing capacity of eugenol.27
This method is based on the reduction of (Fe3+) ferricyanidein stoichiometric excess relative to the antioxidants.22
Briefly, 15-lg/mL concentrations of eugenol in 0.75 mLdistilled water were mixed with 1.25 mL 0.2 M sodiumphosphate buffer (pH, 6.6) and 1.25 mL potassium ferricy-anide [K3Fe(CN)6] (1%). The mixture was incubated at50�C for 20 minutes. The reaction mixture was then acidi-fied with trichloroacetic acid (1.25 mL, 10%). Finally,0.5 mL of FeCl3 (0.1%) was added to this solution, andthe absorbance was measured at 700 nm. Increased absor-bance of the reaction mixture indicates grater reductioncapability.30,31
Cu2+-Cu+ reducing-CUPRAC assay
To determine the cupric ion (Cu2+)–reducing antioxidantcapacity of eugenol, the method proposed by Apak et al.32
and clearly described by Karaman et al.33 was used withslight modifications. To this end, 0.25 mL CuCl2 solution(0.01 M), 0.25 mL ethanolic neocuproine solution(7.5 · 10-3 M), and 0.25 mL CH3COONH4 buffer solution
976 GULCxIN

(1 M) were added to a test tube, followed by mixing with a45-lg/mL concentration of eugenol. Total volume was thenadjusted to 2 mL with distilled water and thoroughly mixed.The tubes were stoppered and kept at room temperature.Absorbance was measured at 450 nm against a reagent blank30 minutes later. Increased absorbance of the reaction mix-ture indicates increased reduction capability.34
DPPH�-scavenging activity
The hydrogen atom or electron-donation ability of eugenolwas measured from the bleaching of the purple ethanol so-lution of DPPH. When a hydrogen atom or electron wastransferred to the odd electron in DPPH�, the absorbance at517 nm decreased proportionally to the increases in non-radical forms of DPPH.35 Free radical–scavenging capacityof eugenol was determined and compared with that of BHA,BHT, a-tocopherol, and Trolox� (Hoffman-LaRoche) byusing the DPPH�, ABTS�+, DMPD�+, and O2
�- radical–scavenging methods. The DPPH� solution is deep violet, andradical-scavenging activity of antioxidant compounds can bemeasured spectrophotometrically at 517 nm by the loss ofabsorbance as the pale yellow nonradical form (DPPH-H) isproduced. The method of Blois,36 previously described byGulcin,37 was used with slight modifications to assess theDPPH� free radical–scavenging capacity of eugenol. TheDPPH radical shows absorbance at 517 nm, but its absorptiondecreases upon reduction by an antioxidant or a radical.27
Briefly, 0.1-mM solution of DPPH� was prepared in ethanol,and 0.5 mL of this solution was added to a 1.5-mL eugenolsolution in ethanol at different concentrations (10–30 lg/mL).These solutions were vortexed thoroughly and incubated inthe dark for 30 minutes. Thirty minutes later, the absorbancewas measured at 517 nm against blank samples lackingscavenger. A standard curve was prepared by using differentconcentrations of DPPH�. The DPPH�-scavenging capacitywas calculated from the calibration curve determined bylinear regression (r2 = 0.9848):
Absorbance (A517)¼ 0:9692 · [DPPH$] · 10� 4 Mþ 0:215
The capability of scavenging the DPPH�radical was cal-culated by using the following equation:
Scavenged DPPH$(%)¼ 1� A517-S
A517-C
� �· 100
where A517-C is the absorbance of control reaction (con-taining all reagents except the test compound) and A517-S isthe absorbance at 517 nm containing the test compound. Theconcentration of eugenol providing 50% inhibition (IC50)was calculated from the graph-plotted inhibition percentageagainst eugenol concentration (lg/mL).36,37 DPPH signifi-cantly decreases upon exposure to radical scavengers.38,39
ABTS�+-scavenging activity
The ABTS radical cation decolorization test is widelyused to assess the antioxidant activity of various substances.
The experiment was carried out by using an improved ABTSdecolorization assay.40 In this method, an antioxidant isadded to a preformed ABTS radical solution, and after afixed time period, the remaining ABTS�+ is quantifiedspectrophotometrically at 734 nm.41 The ABTS�+ was pro-duced by reacting 2 mM ABTS in H2O with 2.45 mM po-tassium persulfate (K2S2O8) and was stored in the dark atroom temperature for 6 hours. The ABTS�+ solution wasdiluted to give an absorbance of 0.750 – 0.025 at 734 nm in0.1 M sodium phosphate buffer (pH, 7.4). Then, 1 mL ofABTS�+ solution was added to 3 mL of eugenol solution inethanol at different concentrations (10–30 lg/mL). The ab-sorbance was recorded 30 minutes after mixing, and thepercentage of radical scavenging was calculated for eachconcentration relative to a blank-lacking scavenger. Theextent of decolorization is calculated as percentage reduc-tion of absorbance. For preparation of a standard curve,different concentrations of ABTS�+ (0.033–0.33 mM) wereused. The ABTS�+ concentration (mM) in the reactionmedium was calculated from the following calibrationcurve, determined by linear regression (r2 = 0.9841):
Absorbance (A734)¼ 4:6788 · [ABTS�þ ]þ 0:199
ABTS�+-scavenging capability of test compounds was cal-culated by using the following equation:
ABTS�þ scavenging effect (%)¼ 1� A734-S
A734-C
� �· 100
where A734-C is the absorbance of a control lacking anyradical scavenger and A734-S is absorbance of the remainingABTS�+ in the presence of scavenger.42,43 The concentra-tion of eugenol providing 50% inhibition (IC50) was cal-culated from the graph-plotted inhibition percentage againsteugenol concentration (lg/mL).37,44,45
DMPD�+-scavenging activity
Finally, antiradical capacity was analyzed by DMPD�+
assay. DMPD radical–scavenging ability of eugenol wasperformed by using the method of Fogliano et al.,46 withslight modifications.47 In the presence of Fe3+, antioxidantcompounds can transfer a hydrogen atom to DMPD�+, re-sulting in a decolorization of the solution, measured by thedecrease in absorbance at 505 nm. DMPD (100 mM) wasprepared by dissolving 209 mg DMPD in 10 mL deionizedwater, and 1 mL of this solution was added to 100 mL 0.1 Macetate buffer (pH, 5.3). The colored radical cation(DMPD�+) was obtained by adding 0.2 mL of a solution of0.05 M ferric chloride (FeCl3). The absorbance of this so-lution, which is freshly prepared daily, is constant up to12 hours at room temperature. Different concentrations ofstandard antioxidants or eugenol (10–30 lg/mL) wereadded in test tubes, and the total volumes were adjusted to0.5 mL with distilled water. Ten minutes later, the absor-bance was measured at 505 nm. One milliliter of DMPD�+
solution was directly added to the reaction mixture, and its
ANTIOXIDANT ACTIVITY OF EUGENOL 977

absorbance was measured at 505 nm. The buffer solutionwas used as a blank sample. The DMPD�+ concentration(mM) in the reaction medium was calculated from the fol-lowing calibration curve, determined by linear regression(r2 = 0.9993):
Absorbance (A505)¼ 0:0088 · [DMPD�þ ]
The scavenging capability of DMPD�+ radical was calcu-lated by using the following equation:
DMPD�þ scavenging effect (%)¼ 1� A505-S
A505-C
� �· 100
where in A505-C is the initial concentration of the DMPD�+
and A505-S is absorbance of the remaining concentration ofDMPD�+ in the presence of eugenol. The concentration ofeugenol providing 50% inhibition (IC50) was calculatedfrom the graph-plotted inhibition percentage against euge-nol concentration (lg/mL).48,49
Statistical analysis
The experimental analyses were performed in triplicate.The data were recorded as means – standard deviations andwere analyzed by SPSS software, version 11.5 for Windows2000 (SPSS Inc.). One-way analysis of variance was per-formed by following the procedures. Significant differencesbetween means were determined by using Duncan multiplerange tests. A P value less than .05 was considered to rep-resent a significant difference, and a P value less than .01represented a very significant difference.
RESULTS
Antioxidant capacity is defined as the ability of a com-pound to inhibit oxidative degradation, such as lipid per-oxidation.50 Lipid peroxidation can be defined as theoxidative deterioration of lipids containing several carbon–carbon double bonds. To determine the possible effects ofeugenol, its affinity to restrict the peroxidation of a linoleicacid emulsion was tested. Oxidation of linoleic acid gener-ates linoleic acid hydroperoxides, which decompose tosecondary oxidation products. The ferric thiocyanate
method measures the amount of peroxide produced duringthe initial stages of oxidation, which are the primary prod-ucts of linoleic acid oxidation. The oxidized products reactwith ferrous ions to form ferric ions, then to blood-redferric thiocyanate. In the presence of antioxidants, oxidationof linoleic acid will be slow. As a result, because of theformation of thiocyonate, the color development will beslow.
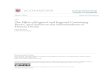
In the present study, eugenol exhibited effective antiox-idant activity in the linoleic acid emulsion system. Table 1and Figure 1 show the effect of 15 lg/mL eugenol onlipid peroxidation of a linoleic acid emulsion: 96.7% (r2 =0.9319). Conversely, BHA, BHT, a-tocopherol, andTrolox exhibited 95.4% (r2 = 0.8482), 99.7% (r2 = 0.7798),84.6% (r2 = 0.9272), and 95.6% (r2 = 0.8511) peroxidation
Table 1. Total Antioxidant Activity (Thiocyanate Method), Fe3+
-Fe2+
–Reducing Ability (Fe3+
-Fe2+
Transformation Method),
and Cu2+
-Cu+–Reducing Ability (by CUPRAC Method) of Eugenol and Standard Compounds
Total antioxidant activity Fe3+-Fe2+–reducing abilitya,b Cu2+-Cu+–reducing abilitya,b
Compound l500a,b r2 l700
a r2 l450a r2
BHA 95.4 0.8482 1.148 0.9011 0.640 0.9214BHT 99.7 0.7798 0.905 0.9764 0.538 0.9622Trolox 95.6 0.8511 0.140 0.7506 0.604 0.9847a-Tocopherol 84.6 0.9272 0.722 0.9581 0.534 0.8886Eugenol 96.7 0.9319 1.180 0.9677 0.762 0.9957
aValues are expressed as absorbance. High absorbance indicates high reducing ability.bAt the same concentration of 45 lg/mL.
BHA, butylated hydroxyanisole; BHT, butylated hydroxytoluene.
0
0,5
1
1,5
2
2,5
0 10 20 30 40 50
Incubation time (h)
Lip
id o
xida
tion
(50
0nm
)
Control
BHA-45 mg/mL
BHT-45 mg/mL
a-Tocopherol-45 mg/mL
Trolox-45 mg/mL
Eugenol-15 mg/mL
FIG. 1. The total antioxidant activity of eugenol (at a concentrationof 15 lg/mL) and standard compounds such as butylated hydro-xyanisole, butylated hydroxytoluene, a-tocopherol, and Trolox at thesame concentration (45 lg/mL) was determined by using the ferricthiocyanate method in the linoleic acid emulsion system. With thismethod, peroxide formation occurred during the oxidation of linoleicacid emulsion, and these compounds oxidized Fe2+ to Fe3+; Fe3+
forms a complex with SCN, with a maximum absorbance at 500 nm.Control samples contained only linoleic acid emulsion and sodiumphosphate buffer. The linoleic acid emulsion was incubated at 37�Cuntil control values reached a plateau. BHA, butylated hydro-xyanisole; BHT, butylated hydroxytoluene.
978 GULCxIN

of linoleic acid emulsion at the same concentration, re-spectively. Peroxidation of linoleic acid emulsion withouteugenol or standard compounds was accompanied by a rapidincrease in peroxides. Consequently, these results clearlyindicate that eugenol had effective and potent antioxidantactivity in the ferric thiocyanate assays.
Furthermore, eugenol had effective reducing power, asdetermined by using the potassium ferricyanide reductionand cupric ions (Cu2+)–reducing methods when comparedwith the standards. To measure the reductive ability of eu-genol, Fe3+-Fe2+ transformation was investigated in thepresence of eugenol by using the method of Oyaizu.29 Asshown in Table 1, eugenol demonstrated powerful, statisti-cally significant Fe3+-reducing ability (r2 = 0.9677; P < .01).The reducing power of eugenol, BHA, BHT, a-tocopherol,and Trolox increased steadily with increasing concentrationof samples. The reducing power of eugenol and the standardcompounds was as follows: eugenol > BHA > BHT > a-tocopherol > Trolox. The results demonstrated that eugenolhad marked ferric ion (Fe3+)–reducing ability and electron-donor properties for neutralizing free radicals by formingstable products. This reducing power was higher than that ofthe standard antioxidants used. The outcome of the reducingreaction is to terminate the radical chain reactions that mayotherwise be very damaging.
The putative CUPRAC method developed by Apaket al.32 was used to determine the antioxidant capacity ofeugenol by the Cu2+-neocuproine reagent as the chromo-genic oxidizing agent. Table 1 shows a cupric ion (Cu2+)–reducing ability of eugenol, and a correlation was ob-served between the cupric ion–reducing ability and eugenolconcentrations (r2 = 0.9957). Cu2+-reducing capability ofeugenol measured by the CUPRAC method was foundto be concentration-dependent (10–30 lg/mL). Cu2+ ion–reducing power of eugenol and standard compounds at thesame concentration (45 lg/mL) was as follows: eugenol‡ Trolox ‡ BHT > BHA ‡ a-tocopherol.
In the DPPH assay, the antioxidants reduced the stableradical DPPH to the yellow diphenyl-picrylhydrazine. Thismethod is based on the reduction of DPPH in alcoholicsolution in the presence of a hydrogen-donating antioxidantbecause of the formation of the nonradical form DPPH-H inthe reaction. DPPH is usually used as a reagent to evaluatefree radical–scavenging activity of antioxidants.29
DPPH is a stable free radical, showing a maximum ab-sorbance at 517 nm. When DPPH radicals encounter aproton-donor substrate such as an antioxidant, the radicalswould be scavenged and the absorbance is reduced.51,52 Thedecrease in absorbance is taken to evaluate the radicalscavenging. Table 2 shows the DPPH radical–scavengingactivity of eugenol and eugenol benzoate at different con-centrations. The results clearly indicate that the eugenolshowed high DPPH free radical activity with the lowest IC50
value (16.06 lg/mL; r2 = 0.9823). The IC50 values for stan-dard compounds (BHA, BHT, a-tocopherol, and Trolox)correspond to 25.51 (r2 = 0.9672), 34.01 (r2 = 0.9857), 33.85(r2 = 0.9962), and 86.93 lg/mL (r2 = 0.7925), respectively.A lower median effective concentration (EC50) value indi-cates higher DPPH free radical–scavenging activity. TheEC50 value depends on the number of DPPH moles reducedby the eugenol during the reaction. DPPH� radical–scav-enging capacity of these samples decreased in the followingorder: eugenol > BHA > a-tocopherol&BHT > Trolox.
All the tested compounds exhibited effective radical cat-ion–scavenging activity. As seen in Table 2, eugenol is aneffective ABTS�+ radical scavenger in a concentration-dependent manner (10–30 lg/mL, r2 = 0.9995). The EC50
value for eugenol in this assay was 7.84 lg/mL. The con-centration of ABTS�+ decreased significantly (P < .01) be-cause of the scavenging capacity at all eugenol concentrations(10–30 lg/mL). Conversely, EC50 values for BHA, BHT,a-tocopherol, and Trolox were 4.68 (r2 = 0.9958), 9.12(r2 = 0.8118), 18.14 (r2 = 0.9910), and 22.80 lg/mL (r2 =0.9410), respectively. The ABTS�+-scavenging effect of eu-genol and standards decreased in the following order:BHA > eugenol > BHT > a-tocopherol > Trolox.
As shown in Table 2, eugenol was an effective DMPD�+
radical scavenger in a concentration-dependent manner (10–30 lg/mL; r2 = 0.9886). EC50 was 10.04 lg/mL for eugenol,63.25 lg/mL for BHA (r2 = 0.9025), and 14.91 lg/mL forTrolox (r2 = 0.8098). The concentration of DMPD�+ de-creased significantly (P < .05) because of the scavengingcapacity at all eugenol concentrations. The main drawbackof the DMPD�+ method is that its sensitivity and repro-ducibility dramatically decreased when hydrophobic anti-oxidants, such as a-tocopherol or BHT, were used.53 LowerEC50 values for DMPD�+ radical–scavenging correspond tohighest DMPD�+ radical–scavenging capacity.54
Table 2. Concentration Required for 50% Scavenging (IC50) activities of Eugenol and Standard Compounds
DPPH� ABTS�+ DMPD�+
Compounds IC50a r2 IC50
a r2 IC50a r2
BHA 25.51 0.9672 4.68 0.9958 63.25 0.9025BHT 34.01 0.9857 9.12 0.8118 – –Trolox 86.93 0.7925 22.8 0.9410 14.91 0.8098a-Tocopherol 33.85 0.9962 18.14 0.9910 – –Eugenol 16.06 0.9823 7.84 0.9995 10.04 0.9886
aThe values are expressed as lg/mL. Lower IC50 values indicate higher radical-scavenging activity.
ABTS�+, 2,20-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid radicals; BHA: butylated hydroxyanisole; BHT, butylated hydroxytoluene; DMPD�+: N,N-
dimethyl-p-phenylenediamine dihydrochloride radicals; DPPH�, 1,1-diphenyl-2-picryl-hydrazyl free radicals.
ANTIOXIDANT ACTIVITY OF EUGENOL 979

DISCUSSION
Phenolics share the same general structure—they arecomposed of an aromatic hydroxyl nucleus and exist in anapproximated number of 8000 in nature.33 So far, plantphenolics constitute one of the major groups of compoundsacting as primary antioxidants or free radical terminators.Plant polyphenols are multifunctional in the sense that theycan act as reducing agents, hydrogen atom donators, andsinglet oxygen scavengers. Certain polyphenols are alsoeffective as antioxidants capable of chelating transitionmetal ions, which may otherwise induce Fenton-type oxi-dation reactions in their free states.33,55
Eugenol, a major phenolic component from clove oil(Eugenia caryophyllata), has demonstrated several biologi-cal activities, such as anti-inflammatory activity by inhibit-ing the enzyme cyclooxygenase-II,56 analgesic activity dueto selective binding at the capsaicin receptor, anti-oxidationactivity,57 and antibacterial activity against both gram-pos-itive and gram-negative microorganisms.58,59 In addition, ithas been widely used as a fragrant and flavoring agent in avariety of food and cosmetic products.60–62 However, athigh concentrations, eugenol has adverse effects, includinginflammatory and allergic reactions, possibly due to theformation of phenoxyl radicals and, subsequently, of qui-nine intermediates, via its pro-oxidant activity.63
Eugenol inhibits neuronal excitotoxic or oxidative injuryand has protective effects against N-methyl-D-aspartate–induced neurotoxicity. The effect of eugenol on lipid per-oxidation and oxidation of low-density lipoprotein wasstudied.64–66 It also protected in vivo inhibition of propa-gation of lipid peroxidation.67 Eugenol reportedly protectednicotine-induced superoxide-mediated oxidative damage inmurine peritoneal macrophages in vitro. In a recent study,eugenol inhibited the activity and gene expression of in-ducible cyclooxygenase in lipopolysaccharide-activatedmouse macrophage cells.68 In addition, inhibition of xan-thine oxidase by eugenol was reported by Rajakumar andRao69 and Jeng et al.70
The present study shows the antioxidant and radical-scavenging mechanism of eugenol by using different in vitrobioanalytical methods. As reported in many studies, theactivities of natural antioxidants in influencing diseases areclosely related to their ability to reduce DNA damage,mutagenesis, carcinogenesis, and inhibition of pathogenicbacterial growth.71 Antioxidant capacity is widely used as ameasure for medicinal bioactive components. In this study,the antioxidant and radical-scavenging activities of eugenolwere compared with those of BHA, BHT, a-tocopherol, andits water-soluble analogue Trolox. These comparisonswere made by using a series of in vitro tests, includingDPPH� scavenging, ABTS�+ scavenging, DMPD�+ scav-enging, total antioxidant activity (by the ferric thiocyanatemethod), and reducing power (by 2 methods).
Various antioxidant assay methods have been developedfor food and biological samples. Lipid peroxidation in bio-logical systems has long been thought to be a toxicologicphenomenon that can lead to various pathologic conse-
quences.72 The resulting lipid hydroperoxides can af-fect membrane fluidity and the function of membraneproteins. In addition, lipid hydroperoxides can undergo iron-mediated, 1-electron reduction, and oxygenation to formepoxyallylic peroxyl radicals. These radicals, in turn, triggera chain reaction of free radical–mediated lipid peroxidation.The end products of lipid peroxidation are reactive alde-hydes, such as 4-hydroxylnonenal and malondialdehyde,many of which are highly toxic to cells.73 In addition, re-active aldehydes generated by lipid peroxidation can attackother cellular targets, such as proteins and DNA, therebypropagate the initial damage in cellular membranes to othermacromolecules. Because lipid hydroperoxides formed inmembranes are important components of ROS generationin vivo, their detoxification appears to be critical for thesurvival of an organism in oxidative stress.74 Therefore,antioxidants play a vital role in inhibition of lipid perox-idation or in protection against cellular damage by freeradicals.
Lipid oxidation consists of a series of free radical–mediated chain reaction processes and is associated withseveral types of biological damage. The ferric thiocyanatemethod measures the amount of peroxide produced duringthe initial stages of oxidation, which is the primary productof lipid oxidation. In this assay, the amount of hydroper-oxides produced from linoleic acid emulsion by auto-oxidation was indirectly measured during the experimentperiod. Ferrous chloride and thiocyanate then react witheach other to produce ferrous thiocyanate by means ofhydroperoxides.75–77
On the other hand, reducing power reflects the electron-donating capacity of bioactive compounds and is associatedwith antioxidant activity. Antioxidants can be reductantsand inactivate oxidants. The reducing capacity of a com-pound can be measured by the direct reduction ofFe[(CN)6]3 to Fe[(CN)6]2. Addition of free Fe3+ to the re-duced product leads to the formation of the intense Perl’sPrussian blue complex, Fe4[Fe(CN-)6]3, which has a strongabsorbance at 700 nm. An increase in absorbance of thereaction mixture would indicate an increase in the reducingcapacity due to an increase in the formation of the complex.Many assays are designed to measure overall antioxidantactivity, or reducing potential, as an indication of a host’stotal capacity to withstand free radical stress. The ferric ion–reducing antioxidant power assay takes advantage of anelectron transfer reaction in which a ferric salt is used as anoxidant.20,47 In this assay, the yellow of the test solutionchanges to various shades of green and blue depending onthe reducing power of antioxidant samples. The reducingcapacity of a compound may serve as a significant indicatorof its potential antioxidant activity.23,28
The present study used the CUPRAC assay, which isbased on reduction of Cu2+ to Cu+ by eugenol. This methodis simultaneously cost-effective, rapid, stable, selective, andsuitable for a variety of antioxidants, regardless of chemicaltype or hydrophilicity. Moreover, it was reported that theresults obtained from in vitro cupric ion (Cu2+)–reducingmeasurements might be more efficiently extended to the
980 GULCxIN

possible in vivo reactions of antioxidants. CUPRAC chro-mogenic redox reaction is carried out at a pH (7.0) close tothe physiologic pH, and the method is capable of measuringthiol-type antioxidants, such as glutathione, and nonproteinthiols, unlike the widely applied ferric-reducing antioxidantpower test, which is nonresponsive to -SH group antioxi-dants.33,78
It has been reported that the main mechanism of action ofphenolic antioxidants was considered to be the scavengingof free radicals, although other mechanisms may be in-volved.79 The radical-scavenging activity of phenolics de-pends on structural characteristics that favor phenolichydrogen donation and the stability of the resulting phe-noxyl radicals. Only when at least a second phenol group isattached to this chain (e.g., rosmarinic acid) does its con-tribution becomes significant. In addition, extension of theconjugation to the carbon chain is a molecular feature thatrequires some attention because it could participate byresonance to the stabilization of the phenoxyl radical.79 Thefree radical chain reaction is widely accepted as a commonmechanism of lipid peroxidation. Radical scavengers maydirectly react with and quench peroxide radicals to terminatethe peroxidation chain reactions and improve the quality andstability of food products.80 Assays based on the use ofDPPH�, ABTS�+, and DMPD�+ radicals are among the mostpopular spectrophotometric methods for determination ofthe antioxidant capacity of foods, beverages, and vegetableextracts. Both chromogens and radical compounds can di-rectly react with antioxidants. Additionally, DPPH�- andABTS�+-scavenging methods have been used to evaluate theantioxidant activity of compounds because of the simple,rapid, sensitive, and reproducible procedures.81
In this study, 3 radical-scavenging methods were used toassess the potential radical-scavenging activities of eugenol:DPPH�, ABTS�+, and DMPD�+. With these methods, it ispossible to determine the antioxidant power of an antioxi-dant by measuring a decrease in the absorbance of DPPH� at517 nm. The structure of eugenol provides a chromophoricsystem that leads to interference in the DPPH� method usedin this study with the 517 nm wavelength, as describedearlier. The absorbance is decreased when DPPH� is scav-enged by an antioxidant through donation of hydrogen toform a stable DPPH radical molecule. In the radical form,this molecule has an absorbance at 517 nm, which disap-pears after acceptance of an electron or hydrogen radicalfrom an antioxidant compound to become a stable dia-magnetic molecule.82
Free radical–scavenging is one of the known mechanismsby which antioxidants inhibit lipid oxidation. This test is astandard assay in antioxidant activity studies and offers ra-pid screening of the radical-scavenging activity of specificcompounds. The antioxidants are believed to intercept thefree radical chain of oxidation and donate hydrogen from thephenolic hydroxyl groups, thereby forming a stable end-product that does not initiate or propagate further oxidationof lipid.80 Reactions with DPPH radicals have been reportedin the literature. Gulcin suggested the best-known mecha-nism for L-carnitine and the DPPH radical.28 L-carnitine has
a carbonyl group, which stabilized a radical formed on a-carbon with conjugation in the L-carnitine molecule. Thiscarboxylate group also had a carbonyl unit. An abstractionof a hydrogen atom from C2 may occur easily.
Kawabata et al. reported the formation of dimers fromgallic and protocatechuic acids after reaction with DPPHradical.83 Brand-Williams et al.84 and Bondet et al.85 stud-ied the possible mechanism of DPPH with BHT, eugenol,and isoeugenol and proposed the intervention of a morecomplex reaction mechanism, eventually involving di-meric species. Dehydrodiisoeugenol occurred via oxidativecoupling of eugenol.86 To confirm observations made onthe role of the side chain, a group of 3 monophenols—isoeugenol, dihydroeugenol, and eugenol—differing in 1double bond or its position in the chain, was also known. Inaddition, conjugation seemed to enhance the antioxidant andradical-scavenging activity of eugenol.79 It is accepted thatthe scavenging mechanism of reaction is abstracting a hy-drogen atom from a phenol donor to give DPPH-H and aphenoxy radical.87 It was reported that eugenol reduces 2 ormore DPPH radicals, despite the availability of only 1 hy-drogen from a hydroxyl group, and different hypotheseshave been proposed to explain the antiradical efficiencies ofthe different monophenolic compounds.87,88 In anotherstudy, coantioxidant behavior of some relevant phenols,such as eugenol and isoeugenol, was studied. Synergism,implying regeneration of a-tocopherol by the coantioxidant,was observed with combination of a-tocopherol/eugenolwith peroxy radicals.89
The dimers with 2 phenolic hydroxyl groups of A, B, andC were present at a very low amount compared with C. Allthese compounds can be originated from the C8-C8 and C5-C5 coupling processes, as hypothesized in Figure 2.86
Eugenol is believed to have an aromatic ring. This phe-nolic group stabilized a radical formed on a-carbon withconjugation in the eugenol molecule. As can be seen inFigure 2, eugenol scavenged the radical on this aromaticring. Similarly, the structure of resveratrol provides achromophoric system that leads to interference in DPPH�. Itis well known that phenolic groups stabilize a radicalformed on phenolic carbon with their resonance structure.Resveratrol has 2 phenolic rings: monophenol and diphenol.An abstraction of a hydrogen atom from monophenolichydroxyl group may occur easily.23
ABTS�+ is applicable for both lipophilic and hydrophiliccompounds. ABTS�+ radicals are more reactive than DPPHradicals, and unlike the reactions with DPPH radical, whichinvolve H atom transfer, the reactions with ABTS�+ radicalsinvolve an electron-transfer process. Generation of theABTS radical cation forms the basis of one of the spectro-photometric methods that have been applied to measure thetotal antioxidant activity of pure substances, aqueous mix-tures, and beverages.47 A more appropriate format for theassay is a decolorization technique in which the radical isgenerated directly in a stable form before the reaction withputative antioxidants.
ABTS�+ was generated by oxidation of ABTS with po-tassium persulfate. This assay is based on the inhibition of the
ANTIOXIDANT ACTIVITY OF EUGENOL 981

absorbance of the radical cation ABTS�+, which has a char-acteristic long-wavelength absorption spectrum showing ab-sorption at 734 nm. The reaction with ABTS�+ was quite fast,and in almost all cases was completed in 0.25 to 0.5 min.Bleaching of a preformed solution of the blue-green radicalcation ABTS�+ has been extensively used to evaluate theantioxidant capacity of complex mixtures and individualcompounds. The reaction of the preformed radical with freeradical scavengers can be easily monitored by following thedecay of the sample absorbance at 734 nm.20,35
The principle of the DMPD�+ assay is that DMPDcan form a stable and colored radical cation (DMPD�+) at anacidic pH and in the presence of a suitable oxidant solu-tion. The ultraviolet visible-light spectrum of DMPD�+
shows a maximum absorbance at 505 nm. Antioxidantcompounds, which can transfer a hydrogen atom toDMPD�+, quench the color, and produce a decolorizationof the solution. This reaction is rapid, and the end point,which is stable, is taken as a measure of antioxidativeefficiency. Therefore, this assay reflects the ability of radical
Eugenyl intermediate radicals
A B
DPPH
DPPH-H
O
OCH3
OH
OCH3
O
OCH3
O
OCH3
O
OCH3
C D
2
Eugenol
Dehydrodieugenol
2
OH
OCH3
OHH3CO H3CO
HO OCH3
OH
12
3
45
6
78
9
O
OCH3HO
OCH3
321
FIG. 2. The proposed mechanism between eugenol and DPPH radicals and formation of dehydrodieugenol.
982 GULCxIN

hydrogen donors to scavenge the single electron fromDMPD�+.27,46
Preliminary experiments show that the choice of oxi-dant solution and the ratio between the concentration ofDMPD�+ and the concentration of the oxidative compoundis crucial for the effectiveness of the method. In fact, for-mation of radical cation is very slow and results in a con-tinuous increase of the absorbance. The best results wereobtained with FeCl3, which gives a stable colored solutionup to a final concentration of 0.1 mM. Moreover, thismethod ensures low cost and highly reproducible analysis.26
The DMPD assay is particularly suitable for hydrophilicantioxidants but is less sensitive to hydrophobic bioactivecompounds; the opposite applies for the other 2 tests. Incontrast to the ABTS procedure, the DMPD�+ methodguarantees a very stable end point. This is particularly im-portant when a large-scale screening is required. It has beenreported that the main drawback of the DMPD�+ method isthat its sensitivity and reproducibility dramatically de-creased when hydrophobic antioxidants, such as a-tocoph-erol or BHT, were used. Hence, these standard antioxidantcompounds were not used in this antiradical assay.
CONCLUSION
The principle of antioxidant activity is based on theavailability of electrons to neutralize any free radicals. Inaddition, antioxidant activity is related to the number and thenature of the hydroxylation pattern on the aromatic ring. It isgenerally assumed that the ability to act as hydrogen donorand the inhibition of oxidation are enhanced by the increasein the number of hydroxyl groups in the phenol ring. Eugenolhas an aromatic ring. In this study, Fe3+-Fe2+– and Cu2+-Cu+–reducing abilities and DPPH�-, ABTS�+-, andDMPD�+-scavenging activities were adopted to evaluate theantioxidant activities of eugenol at different concentrations.The results were compared with BHA, BHT, a-tocopherol,and Trolox, which were used as standard antioxidants. Inaddition, the possible mechanisms between antioxidantactivity of eugenol and its structure were clarified.
ACKNOWLEDGMENT
This study was partially supported by the Research Fundof the Ataturk University. The author would like to expressgratitude to the Research Fund of the Ataturk University forthe funding of Project (BAP-2008/75).
AUTHOR DISCLOSURE STATEMENT
The author reports no conflicts of interest.
REFERENCES
1. Szabadics J, Erdelyi L: Pre-and postsynaptic effects of eugenol
and related compounds on Helix pomatia L. Acta Biol Hung
2000;51:265–273.
2. Tai KW, Huang FM, Huang MS, Chang YC: Assessment of the
genotoxicity of resin and zinc-oxide eugenol-based root canal
sealers using an in vitro mammalian test system. J Biomed.Mat
Res 2002;59:73–77.
3. Chang MC, Uang BJ, Wu HL, et al.: Inducing the cell cycle
arrest and apoptosis of oral KB carcinoma cells by hydorox-
ychavicol: roles of glutathione and reactive oxygen species. Br J
Pharmacol 2002;135:619–630.
4. Francis LE, Wood DR: Dental Pharmacology and Therapeutics.
Philadelphia, W.B. Saunders, 1961.
5. Frisch J, Bhaskar SN: Tissue response to eugenol containing
periodontal dressings. J Peridontol 1968;38:402–408.
6. Dallmeier K, Carlini EA: Anesthetic, hypothermic, myorelaxant
and anticonvulsant effects of synthetic eugenol derivatives and
natural analogues. Pharmacology 1981;22:113–127.
7. Brodin P, Roed A, Effects of eugenol on rat phrenic nerve and
phrenic nerve diaphragm preparation. Arch Oral Biol 1984;29:
611–615.
8. Sen P, Maiti PC, Puri S: Mechanism of antistress activity of
Ocimum sanctum Linn, eugenol and Tinospora malabaria in
animals. Ind J Exp Biol 1992;30:592–596.
9. Walsh SE, Maillard JY, Russell AD, et al.: Activity and mech-
anisms of action of selected biocidal agents on grampositive and
-negative bacteria. J Appl Microbiol 2003;94:240–247.
10. Chami N, Bennis S, Chami F, Aboussekhra A, Remmal A: Study
of anticandidal activity of carvacrol and eugenol in vitro and
in vivo. Oral Microbiol Immunol 2005;20:106–111.
11. Lee SJ, Han JI, Lee GS, et al.: Antifungal effect of eugenol and
nerolidol against Microsporum gypseum in a guinea pig model.
Biol Pharm Bull 2007;30:184–188.
12. Rompelberg CJ, Evertz SJ, Bruijntjes-Rozier GC, van den Heu-
vel PD, Vehhagen H: Effect of eugenol on the genotoxicity of
established mutagens in the liver. Food Chem Toxicol 1996;34:
33–42.
13. Zheng GQ, Kenney PM, Lam LK. Sesquiterpenes from clove
(Eugenia caryophyllata) as potential anticarcinogenic agents. J
Nat Prod 1992;55:999–1003.
14. Ozeki M: The effects of eugenol on the nerve and muscle in
crayfish. Comp Biochem Physiol 1975;50C:183–191.
15. Pandey BN, Lathika KM, Mishra KP: Modification of radiation-
induced oxidative damage in liposomal and microsomal mem-
brane by eugenol. Rad Physics Chem 2006;75:384–391.
16. FAO: Evaluation of certain food additives and contaminants.
Technical Report Series No. 20. Geneva, Switzerland, FAO/
WHO Expert Committee on Food Additives, 1982.
17. Cadenas E: Biochemistry of oxygen toxicity. Ann Rev Biochem
1989;58:79–110.
18. Gulcin _I, Mshvildadze V, Gepdiremen A, Elias R: Antioxidant
activity of a triterpenoid glycoside isolated from the berries of
Hedera colchica: 3-O-(b-D-glucopyranosyl)-hederagenin. Phyt-
other Res 2006;20:130–134.
19. Gulcin _I: Antioxidant and antiradical activities of L-Carnitine.
Life Sci 2006;78:803–811.
20. Gulcin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC: Poly-
phenol contents and antioxidant activity of lyophilized aqueous
extract of propolis from Erzurum, Turkey. Food Chem Toxicol
2010;48:2227–2238.
21. Gulcin _I: Antioxidant activity of caffeic acid (3,4-dihydrox-
ycinnamic acid). Toxicology 2006;217:213–220.
22. Gulcin _I: Comparison of in vitro antioxidant and antiradical ac-
tivities of L-tyrosine and L-Dopa. Amino Acids 2007;32:431–
438.
ANTIOXIDANT ACTIVITY OF EUGENOL 983

23. Gulcin _I: Antioxidant properties of resveratrol: A structure-ac-
tivity insight. Innov Food Sci Emerg 2010;11:210–218.
24. Gulcin _I, Dasxtan A: Synthesis of dimeric phenol derivatives and
determination of in vitro antioxidant and radical scavenging ac-
tivities J Enzym Inhib Med Chem. 2007;22:685–695.
25. Gulcin _I, Berashvili D, Gepdiremen A: Antiradical and antioxi-
dant activity of total anthocyanins from Perilla pankinensis
decne. J Ethnopharmacol 2005;101:287–293.
26. Gulcin _I: Measurement of antioxidant ability of melatonin and
serotonin by the DMPD and CUPRAC methods as trolox
equivalent. J Enzym Inhib Med Chem 2008;23:871–876.
27. Ak T, Gulcin _I: Antioxidant and radical scavenging properties of
curcumin. Chem Biol Interact 2008;174:27–37.
28. Gulcin _I: Antioxidant and antiradical activities of L-Carnitine.
Life Sci 2006;78:803–811.
29. Oyaizu M: Studies on product of browning reaction prepared
from glucose amine. Jpn J Nut 1986;44:307–315.
30. Buyukokuroglu ME, Gulcin _I, Oktay M, Kufrevioglu O_I: In vitro
antioxidant properties of dantrolene sodium. Pharmacol Res.
2001;44:491–495.
31. Oktay M, Gulcin _I, Kufrevioglu O_I: Determination of in vitro
antioxidant activity of fennel (Foeniculum vulgare) seed extracts.
Lebensm Wissen Technol 2003;36:263–271.
32. Apak R, Guclu K, Ozyurek M, Karademir SE, Ercal E:
The cupric ion reducing antioxidant capacity and polypheno-
lic content of some herbal teas. Int J Food Sci Nut 2006;57:292–
304.
33. Karaman Sx, Tutem E, Basxkan KS, Apak R: Comparison of total
antioxidant capacity and phenolic composition of some apple
juices with combined HPLC-CUPRAC assay. Food Chem
2009;120:1201–1209.
34. Talaz O, Gulcin _I, Goksu S, Saracoglu N: Antioxidant activity of
5,10-dihydroindeno[1,2-b]indoles containing substituents on di-
hydroindeno part. Bioorg Med Chem 2009;17:6583–6589.
35. Gulcin _I, Kirecci E, Akkemik E, Topal F, Hisar O: Antioxidant
and antimicrobial activities of an aquatic plant: Duckweed
(Lemna minor L.). Turk J Biol 2010;34:175–188.
36. Blois MS: Antioxidant determinations by the use of a stable free
radical. Nature 1958;26:1199–1200.
37. Gulcin _I: The antioxidant and radical scavenging activities of
black pepper (Piper nigrum) seeds. Int J Food Propert. 2005;56:
491–499.
38. Gulcin _I, Elmastas M, Aboul-Enein HY: Determination of anti-
oxidant and radical scavenging activity of basil (Ocimum basi-
licum) assayed by different methodologies. Phytother Res 2007;
21:354–361.
39. Gulcin _I, Tel AZ, Kirecci E: Antioxidant, antimicrobial, anti-
fungal and antiradical activities of Cyclotrichium niveum (Boiss.)
Manden and Scheng. Int J Food Propert 2008;11:450–471.
40. Re R, Pellegrini N, Proteggente A, et al.: Antioxidant activity
applying an improved ABTS radical cation decolorization assay.
Free Radical Bio Med. 1999;26:1231–1237.
41. Gulcin _I, Elias R, Gepdiremen A, Chea A, Topal F: Antioxidant
activity of bisbenzylisoquinoline alkaloids from Stephania ro-
tunda: Cepharanthine and fangchinoline. J Enzym Inhib Med
Chem 2010;25:44–53.
42. Gulcin _I, Elias R, Gepdiremen A, Taoubi K, Koksal E: Anti-
oxidant secoiridoids from fringe tree (Chionanthus virginicus L.).
Wood Sci Technol 2009;43:195–212.
43. Koksal E, Gulcin _I, Ozturk Sarıkaya SB, Bursal E: On the in vitro
antioxidant activity of silymarin. J Enzym Inhib Med Chem
2009;24:395–405.
44. Gulcin _I, Sxat _IG, Beydemir Sx, Elmastasx M, Kufrevioglu O_I:Comparison of antioxidant activity of clove (Eugenia car-
yophylata Thunb) buds and lavender (Lavandula stoechas L.).
Food Chem 2004;87:393–400.
45. Gulcin _I, Beydemir Sx, Alici HA, Elmastasx M, Buyukokuroglu
ME: In vitro antioxidant properties of morphine. Pharmacol Res
2004;49:59–66.
46. Fogliano V, Verde V, Randazzo G, Ritieni A: Method for mea-
suring antioxidant activity and its application to monitoring the
antioxidant capacity of wines. J Agric Food Chem 1999;47:
1035–1040.
47. Gulcin _I: Antioxidant activity of L-adrenaline: an activity-
structure insight. Chem Biol Interact 2009;179:71–80.
48. Buyukokuroglu ME, Gulcin _I: In vitro antioxidant and antiradical
properties of Hippophae rhamnoides L. Phcog Mag 2009;4:189–
195.
49. Gulcin _I, Elias R, Gepdiremen A, Boyer L: Antioxidant activity
of lignans from fringe tree (Chionanthus virginicus L.). Eur Food
Res Technol 2006;223:759–767.
50. Roginsky V, Lissi EA: Review of methods to determine chain-
breaking antioxidant activity in food. Food Chem 2005;92:235–
254.
51. Gulcin _I, Kufrevioglu O_I, Oktay M, Buyukokuroglu ME: Anti-
oxidant, antimicrobial, antiulcer and analgesic activities of nettle
(Urtica dioica L.). J Ethnopharmacol 2004;90:205–215.
52. Elmastas M, Gulcin _I, Isxıldak O, et al.: Antioxidant capacity of
bay (Laurus nobilis L.) leave extracts. J Iran Chem Soc 2006;3:
258–266.
53. Elmastas M, Turkekul _I, Ozturk L, et al.: The antioxidant activity
of two wild edible mushrooms (Morchella vulgaris and Morch-
ella esculanta). Comb Chem High T Scr 2006;9:443–448.
54. Gulcin _I, Elias R, Gepdiremen A, Boyer L, Koksal E: A
comparative study on the antioxidant activity of fringe tree
(Chionanthus virginicus L.) extracts. Afr J Biotechnol 2007;6:
410–418.
55. Rice-Evans CA, Miller NJ, Paganga G: Structure-antioxidant
activity relationships of flavonoids and phenolic acids. Free
Radic Bio Med 1996;20:933–956.
56. Son KH, Kwon SY, Kim HP, Chang HW, Kang SS: Constituents
of Syzigium aromaticum Merr. et Perry. Nat Prod Sci 1998;4:
263–267.
57. Ou HC, Chou FP, Lin TM, Yang CH, Sheu WH: Protective
effects of eugenol against oxidized LDL-induced cytotoxicity
and adhesion molecule expression in endothelial cells. Food
Chem Toxicol 2006;44:1485–1495.
58. Kalemba D, Kunicka A: Antibacterial and antifungal properties
of essential oils. Curr Med Chem 2003;10:813–829.
59. Laekeman SM, Hoof VL, Haemers A, et al.: Eugenol A valuable
compound for in vitro experimental research and worthwhile for
further in vivo investigation. Phytother Res 1990;4:90–96.
60. Atsumi T, Iwakura I, Fujisawa S, Ueha T: Reactive oxygen
species generation and photo-cytotoxicity of eugenol in solutions
of various pH. Biomaterials 2001;22:1459–1466.
61. Fujisawa S, Atsumi T, Kadoma Y, Sakagami H: Antioxidant and
prooxidant action of eugenol-related compounds and their cyto-
toxicity. Toxicology 2002;177:39–54.
984 GULCxIN

62. Ito M, Murakami K, Yoshino M: Antioxidant action of eugenol
compounds: Role of metal ion in the inhibition of lipid perox-
idation Food Chem Toxicol 2005;43:461–466.
63. Baratt MD, Basketter DA, Roberts DW: Skin sensitization to
eugenol and isoeugenol in mice: possible metabolic pathways
involving ortho-quinone and quinone methide intermediates.
Chem Res Toxicol 1997;10:335–343.
64. Murakami K, Ito M, Htay HH, et al.: Antioxidant and prooxidant
actions of gallic acid derivatives: effect on metal-dependent
oxidation of lipids and low density lipoprotein. Biomed Res
2000;21:291–296.
65. Murakami K, Ito M, Htay HH, Tsubouchi R, Yoshino M: Anti-
oxidant effect of capsaicinoids on the metal-catalyzed lipid
peroxidation. Biomed Res 2001;22:15–17.
66. Murakami K, Tanemura Y, Yoshino M: Dipicolinic acid prevents
the copper-dependent oxidation of low density lipoprotein. J Nut
Biochem 2003;14:99–103.
67. Naidu KA: Eugenol: an inhibitor of lipoxygenase-dependent li-
pid peroxidation. Leukot Essential Fatty Acids 1996;53:381–383.
68. Kim SS, Oh OJ, Min HY, et al.: Eugenol suppresses cycloox-
ygenase-2 expression in lipopolysaccharide-stimulated mouse
macrophage RAW264.7 cells. Life Sci 2003;73:337–348.
69. Rajakumar DV, Rao MV: Dehydrozingerone and isoeugenol as
inhibitors of lipid peroxidation and as free radical scavengers.
Biochem Pharmacol 1993;46:2067–2072.
70. Jeng JH, Hahn LJ, Lu FJ, Wang YJ, Kuo MYP: Eugenol triggers
different pathobiological effects on human oral mucosal fibro-
blasts J Dent Res 1994;73:1050–1055.
71. Roginsky V, Lissi EA: Review of methods to determine chain-
breaking antioxidant activity in food. Food Chem 2005;92:235–
254.
72. Hochstein P, Atallah AS: The nature of oxidants and antioxidant
systems in the inhibition of mutation and cancer. Mutat Res
1988;202:363–375.
73. Yu BP, Yang R: Critical evaluation of the free radical theory of
aging. A proposal for the oxidative stress hypothesis. Ann N Y
Acad Sci 1996;786:1–11.
74. Dargel R: Lipid peroxidation—a common pathogenetic mecha-
nism? Exp Toxicol Pathol 1992;44:169–181.
75. Inatani R, Nakatani N, Fuwa H: Antioxidative effect of the
constituents of rosemary (Rosemarinus officinalis L.) and their
derivatives. Agric Biol Chem 1983;47:521–528.
76. Gulcin _I, Beydemir Sx, Sxat _IG, Kufrevioglu O_I: Evaluation of
antioxidant activity of cornelian cherry (Cornus mas L.). Acta
Aliment Hung 2005;34:193–202.
77. Koksal E, Gulcin _I: Antioxidant activity of cauliflower (Brassica
oleracea L.). Turk J Agric For 2008;32:65–78.
78. Gulcin _I, Mshvildadze V, Gepdiremen A, Elias R: Antioxidant
activity of saponins isolated from ivy: a-Hederin, hederasaponin-
C, hederacolchiside-E and hederacolchiside F. Planta Med.
2004;70:561–563.
79. Nenadis N, Boyle S, Bakalbassis EG, Tsimidou M: An experi-
mental approach to structure-activity relationships of caffeic and
dihydrocaffeic acids and related monophenols. JAOCS 2003;80:
451–458.
80. Soares JR, Dins TCP, Cunha AP, Ameida LM: Antioxidant ac-
tivity of some extracts of Thymus zygis. Free Radical Res
1997;26:469–478.
81. Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil
JA: Free-radical scavenging capacity and antioxidant activity of
selected plant species from the Canadian prairies. Food Chem
2004;84:551–562.
82. Matthaus B: Antioxidant activity of extracts obtained from res-
idues of different oilseeds. J Agric Food Chem 2002;50:3444–
3452.
83. Kawabata J, Okamoto Y, Kodama A, Makimoto T, Kasai T:
Oxidative dimers produced from protocatechuic and gallic esters
in the DPPH radical scavenging reaction. J Sci Food Agric
2002;50:5468–5471.
84. Brand-Williams W, Cuvelier ME, Berset C: Use of a free radical
method to evaluate antioxidant activity. Lebensm Wissen Technol
1995;28:25–30.
85. Bondet V, Brand-Williams W, Berset C: Kinetics and mecha-
nisms of antioxidant activity using the DPPH free radical
method. Lebensm Wissen Technol 1997;30:609–615.
86. Bortolomeazzi R, Verardo G, Liessi A, Calle A: Formation
of dehydrodiisoeugenol and dehydrodieugenol from the reac-
tion of isoeugenol and eugenol with DPPH radical and their role
in the radical scavenging activity. Food Chem. 2010;118:256–
265.
87. Mastelic J, Jerkovic I, Bla�zevic I, et al.: Comparative study on
the antioxidant and biological activities of carvacrol, thymol, and
eugenol derivatives. J Agric Food Chem 2008;56:3989–3996.
88. Brandwilliams W, Cuvelier ME, Berset C: Use of free radicals
method to evaluate antioxidant activity. Lebensm Wiss.-Technol
1995;28:25–30.
89. Kadoma Y, Ishihara M, Fujisawa S: A quantitative approach to
the free radical interaction between alpha-tocopherol and the
coantioxidants eugenol, resveratrol or ascorbate. In Vivo 2006;
20:61–67.
ANTIOXIDANT ACTIVITY OF EUGENOL 985

This article has been cited by:
1. Yasin Çetinkaya, Hülya Göçer, Abdullah Menzek, #lhami Gülçin. 2011. Synthesis and Antioxidant Properties of (3,4-Dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and Its Derivatives. Archiv der Pharmazie n/a-n/a. [CrossRef]
Related Documents