EIAR & Dow Leadership in Action Final Report August 13-15 2014 Trip Overview Lab Audit Findings Prioritization & Multigenerational Plan John DiMuro, Matt Miller, David Stenhouse, Leo Hu, & LeAnn Bruns October 7, 2014

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

EIAR & Dow Leadership in Action Final Report
August 13-15 2014
Trip Overview
Lab Audit Findings
Prioritization & Multigenerational Plan
John DiMuro, Matt Miller, David Stenhouse, Leo Hu, & LeAnn Bruns
October 7, 2014

Table of Contents
Trip Overview ................................................................................................................................................ 3
Agenda ...................................................................................................................................................... 3
Attendees .................................................................................................................................................. 5
Project Overview ....................................................................................................................................... 6
Audit Tool Overview ................................................................................................................................. 6
Change Management Overview ............................................................................................................. 18
Establishment of an Interdisciplinary Executive Team ........................................................................... 19
Summary of Audit Findings ..................................................................................................................... 20
Lab Audit Details ......................................................................................................................................... 23
Prioritization and Multi-Generational Plan ................................................................................................. 30
Multi-Generation Plan ............................................................................................................................ 31
Key concepts for the Interdisciplinary Executive team to consider ....................................................... 32
Executing and Implementing Change – Making it “stick” ....................................................................... 32
More Resources ...................................................................................................................................... 33

Trip Overview
Agenda: August 13-15, 2015
Time table Program Responsible When
Day-1 (9:00-9:45)
Welcoming of DOW team to EIAR
EIAR team (Solomon A.)
13 August 2014
(9:45 -10:00) Keynote speech by DDG of EIAR
(Dr. Aduja W.)
(10:00 - 10:15)
Short description on Ethiopia (the country)
EIAR team (Gelila A.)
(10:15 - 10:30)
EIAR profile
(Derese T.)
(10:30 - 11:00)
Coffee break
(11:00 - 12:00) Short brief about DOW DOW team
(12:00 - 01:00) Description on the state of EIAR Laboratories (Part 1)
EIAR team (Solomon A.)
(01:00 - 02:00) Lunch
EIAR Cafeteria
(02:00 - 02:30)
AQRL statues Description on (in the process of accreditation
EIAR team (Gelila A.)
(02:30 - 03:30) Practical visit to the AQRL:[all formats, working procedures and working instructions will be reviewed]
AQRL- team
(03:30 - 04:00) Coffee break
(04:00 -05:00)
General discussion and feedback
DOW and EIAR team
Day -2 (09:00-10:00)
Safety Presentation
DOW team
14 August, 2014
(10:00 - 11:00)
Dow team to use Global Analytical Lab tool to train EIAR - AQRL
DOW team
(11:00 - 11:30)
Coffee break
(11:30 - 01:00) Dow team to use Global Analytical Lab tool to train EIAR - AQRL
(01:00 - 02:00) Lunch

(02:00 - 03:30)
Dow team to use Global Analytical Lab tool to audit EIAR - AQRL
DOW team
(03:30 - 04:00)
Coffee break
(04:00 - 05:00) Dow team to use Global Analytical Lab tool to audit EIAR AQRL
Day -3 (09:00 - 11:00)
Debrief/results review and DOW experience sharing in managing Laboratories
DOW team August 15, 2014 8:30-11:30
(11:00 - 11:30) Coffee break
(11:30 -01:00) Presentation from Dow on Prioritization/Multigenerational Plan /Management of Change
DOW team and EIAR team
(01:00 - 02:00) Lunch
(02:00 - 4:00)
Seating milestones/ schedule that EIAR will follow in implementing DOWs experience
DOW and EIAR team
(04:00 - 05:00)
Coffee break & closing of training

Attendees: EIAR participants:
1. Dr. Fentahun Mengistu, director general of EIAR 2. Dr. Adugna Wakijira, deputy director general of EIAR 3. Solomon Abate, director for Agricultural and Nutritional Research Laboratories 4. Gelila Asaminew, lab head of Agricultural Quality Research Laboratory @ HQ. EIAR team
member in the project 5. Bilatu Agza, researcher in AQRL. EIAR team member in the project 6. Quality management Representatives of Research Centers (listed below) 7. Laboratory heads of Research centers (listed below)
Addis Ababa Mr. Belatu Agza
Mrs. Gelila Asamenew Mr. Dinka Mulugeta Mr. Samuel Mesfin
Mr. Hailu Reta Mr. Yibrah Amare
Mr. Aytenew Abitew
Holleta Agricultural Research Center Mr. Dereje Fikadu Mr. Zerihun Assefa
Wondo Genet Agricultural Research Center
Mr. Beriso Measo Mr. Beemnet Mengesha
Melkassa Agricultural Research Center
Mr. Kebede Dinkecha
Debre Zeit Agricultural Research Center Mr.MohamedYimam
Mr. Melese Menaleshewa
Jimma Agricultural Research Center Mr. Tesfu Kebede
Werer Agriclutural Research Center
Mr. Mulate Zerihun

Dow Chemical attendees:
1. John DiMuro 2. Leo Hu 3. Matt Miller 4. David Stenhouse 5. LeAnn Bruns
Project Overview: Through discussions with EIAR leadership, it was determined that the key project deliverable was a description of what a “world class” laboratory management program consisted of, and recommendations on how EIAR could establish such a program. EIAR leadership also wished to receive guidance on how disparate labs could be unified through common work processes and procedures. The project was therefore designed with two major elements to achieve this vision. The core of the project was an Audit Tool, which not only can be used to perform a gap assessment on individual labs, but which itself describes the management systems necessary to achieve world-class laboratory management practices. It highlights actions and programs that, if implemented consistently throughout the EIAR analytical labs, would set EIAR on the path to world-class laboratory management. The second element of this project was recommendations on how to use the tool as a platform for change and lab unification. Execution of EIAR’s strategy is critical, especially considering the multiple labs spread over a wide geographical area. This report highlights some key concepts discussed during the project, and describes how the Audit Tool can be used as a means for the execution of both lab improvements and EIAR’s lab unification strategy.
Audit Tool Overview: A key deliverable for the Dow/EIAR partnership was a gap and performance assessment of their labs from an analytical quality perspective. After consultation with company experts, discussion with the Analytical Technology Center uncovered a tool used within Dow to understand gaps within Analytical labs. This tool is named the “Global Analytical Performance Scorecard.” The tool is designed to evaluate laboratories with the purpose of setting expectations and measuring performance against ideal analytical practices. The audits occur across ten different dimensions, consisting of additional individual elements, and are listed below: Enablers: these are broad organizational items that promote people/client/instrument satisfaction and cohesiveness

Processes: items around how work is agreed to, planned, and executed Calibration: instrument calibration and standards Preventive Maintenance: keeping instruments working and tracking repairs Training: ensuring people are qualified and have knowledge they need Analytical Instrument Qualification: ensuring instruments can work as needed Statistical Quality Control: tracking performance of equipment Standard Operating Procedures: uniform execution of tests and processes Unplanned Events Management: planning for the unknown before it happens EH&S: making sure people are working safely Best Practices: items to ensure high performing teams and tests Each element has audit criteria that provide guidance as to what level of performance is needed to meet a rating of 0, 3, 6 or 9. It’s also possible to adjust the weighting of each individual element so pre-determined emphases may be leveraged. There was also a list of questions developed for each dimension to facilitate obtaining data to judge each element. Paired with this are individual pieces of data that auditors should look for in order to judge each element. The tables below are the questions and audit evidence for each dimension.
Table 1: Enablers dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
<100% of the direct report
workgroup members have an
Employee Development Plan (EDP)
in place.
Everyone has an EDP Same as 3.
There's a review process in place
for all the EDPs to ensure they are
still relevent
Same as 6.
Share the succession plan for
current leadership/expertise-
requiring roles
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A Need to share the data and the
rating scale so that the 70% is
understood. Questions need to be
challenging enough to be an arbiter
of performance.
Same as 3 - but 85% Same as 6 - but 100%
Element
Score 0 3 6 9
Evidence I am
Looking For
List of lab equipment and list of
MET showing that <50% is being
met
Same as 0, but 50-75% Same as 3, but >75% Same as 6, but 100% and there's
management-approved variances
that can be shown for non-
conformances.
1) Can you show me an employee development plan for at least 3 different employees in 3 different roles?
2) How do you track the review of EDPs? How do you hand these off to new leaders?
3) What's the process for getting client satisfaction survey data?
4) Can you share some results?
5) How often do you collect employee satisfaction data - what drove that decision?
6) How do you use the data to improve your service/performance?
7) What's the process/procedure for determining MET? Can we see the list?
8) Can you share your MET standard documents (procedures)?
9) How do you reconcile different equipment at different sites for the same task?
10) Can we verify that a MET procedure corresponds to the procedure/equipment in the lab? [Pick 4 METs/4 procedures]
Employee Development Plans (EDP's)
Client Satisfaction
Most Effective Technology (MET) standards Implemented

Table 2: Processes dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A There's some discussion, but no
formal document, about testing
plans for customers
Written agreement about testing
exists and contains commitments
for turnaround and priorities of
samples
Same as 6, and the service
agreement is reviewed annually
and is signed-off on by lab and
customer leadership.
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A There's some understand of what a
non-routing request would be and
that it needs to be handled
differently than routine work.
There's a documented process for
non-routing work and this process
contains verbiage around assessing
value of these requests so they can
be prioritized against normal work.
Same as 6, and there's historical
data kept on these requests.
There's also a clear record of
improvement decisions based on
these data.
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A A sample plan with customers Same as 3, but there is some review
of the document every 3 years at a
minimum.
Same as 6, but there is a process for
having a review of the agreement
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A There's a documented sample data
storage process. Samples can be
logged into a system.
Same as 3, and there's evidence of
improvement projects based upon
reviewing this system
Same as 6, and there's a formal
continuous improvement strategy
and the sample data are
electronically mined
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A Samples are logged in and data are
storged, no productivity
measurements
Same as 3, and there's a
productivity analysis
Same as 6, and there's a record of
actions taken via the productivity
analysis
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A There's a 3-5 year capital plan and
there's proof of annual review
Same as 3, and there's a readily
available instrument inventory that
can be shared.
Same as 6, and there's leveraging of
the capital plan into the purchasing
process to get discounts
Element
Score 0 3 6 9
Evidence I am
Looking For
N/A Documented process for
communicating/managing change
Same as 3, but there's an electronic
tool for communicating and
managing change
Same as 6, but there's a history of
auditing the process for
improvement opportunties
1) Can you share some service-level agreements with various other organizations, if they exist?
2) Can you share the process for handling non-routine requests to ensure that good data are being provided?
3) What is the sample plan for the various projects which require more routine testing?
4) How do you store data about samples and data - can you show this system? Can you show some results? What system do you have to track
efficiency?
5) Can you share your inventory and future capital purchase plans?
6) How do you communicate changes in procedures or updates to MET to the organization?
Management of Change (MOC) Process
Sample and Data Management Process
Sample and Work Load Management
Lab Inventory & Capital Plan
Service Level Agreements (SLA)
Non-Routine Request Process
Sample Plan

Table 3: Calibration dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No schedule Documentation on calibration for
all equipment
Proof of fully followed procedures
on calibrations for all equipment
Same as 6, but there's an automatic
schedule for calibrations that
prompts the tests. SQC data is used
to help determine calibration
frequency.
Element
Score 0 3 6 9
Evidence I am
Looking For
No documentation There's detailed instructions on
calibration procedures on <50% of
equipment
Same as 3, but 100% Same as 6, but the procedures also
have guidance on what to do if
there's failures of calibration
Element
Score 0 3 6 9
Evidence I am
Looking For
Required external calibrations are
not performed
Vendor does appropriate
calibrations, but there's no proof or
certification of the vendors
The vendor provides
documentation on traceable
standards and documentation on
what alterations were made to the
instruments
Same as 6, but there's additional
review by a lab SME and there's an
assessment of previous data quality
if there's poor calibration
Element
Score 0 3 6 9
Evidence I am
Looking For
Test materials are expired or audit
data says expired materials were
used
All test materials have expiration
dates that are in the future or
analytical data proving they are OK
if expired.
Same as 3, but there's procedures
for testing standards upon arrival
Same as 6, and all the standards are
NIST (or similar) traceable to be
certified/primary references.
Element
Score 0 3 6 9
Evidence I am
Looking For
No records Calibration data is electronically
stored
Same as 3, including "as found" and
"as left" data along with the raw
calibration data exist.
Same as 6, and the
current/appropriately historic data
are obtainable via a LIMS system
6) Where do you keep your calibrations procedures? Can we see them?
5) Do you have a calibration procedure, and can see records for X instrument calibrations?
2) What kind of materials are used as standards for your calibrations - where do you obtain these from?
3) Can you show me your calibration records for select equipment?
4) Can you show me data on what happens when an instrument fails calibration checks?
7) Can you show me records on vendor calibrations? Is there a sign-off for a EIAR lab SME?
8) Can we see your standards and reagents? What do the expiration dates look like - check this in person?
Standards and Reagents
Calibration Records/Reports
Calibration and Calibration Verification Schedules
Calibration and Calibration Verification Instructions (Internal calibrations)
Calibration by External Vendors

Table 4: Preventive Maintenance dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No documentation There's an informal strategy with
limited documentation
There's a formal maintenance
strategy that's documented with a
priority for certain critical
instruments
Same as 6, and there's an FMEA/risk
analysis for critical components.
There's a formal review to ensure
the strategy is implemented.
Element
Score 0 3 6 9
Evidence I am
Looking For
No procedures Some PM procedure
documentation
All PM procedures are documented Same as 6, and there's details in the
procedures about how to document
the PM
Element
Score 0 3 6 9
Evidence I am
Looking For
No schedule There's a PM schedule for >33% of
instruments
There's a PM schedule for all
instruments
There's a PM schedule with
automatic triggers and a record
proving full PM compliance
Element
Score 0 3 6 9
Evidence I am
Looking For
No spare parts list All quality critical devices have a
spare parts list
Same as 3, and there's enough
inventory to prevent a PM on
critical equipment
Same as 6, and there's a strategic
evaluation of what spare parts
need to be on hand for unexpected
breakdown of the most critical
equipment
Element
Score 0 3 6 9
Evidence I am
Looking For
No records Paper records (logbook) of PM
activities
Electronic records of PM activities Same as 6, and the historic data is
retrievable for understanding
trends
1) Can you show the maintenance records for your equipment?
Do you have a procedure for determining maintenance strategy? Does it review risks of not performing the tests?
Do you have PM procedures? Can we see them?
2) How do you determine the PM schedule for your equipment? Can you share the schedule?
Do you have records of the PMs actually being performed? Can we see them?
3) Do you keep a formal inventory of spare parts?
4) Do you have a determination of what equipment or parts are critical to quality and what is not? What is that and can we see it for your critical
equipment?
Equipment Spare Parts Inventory
Maintenance Records/Reports
Maintenance Strategy
Preventive Maintenance (PM) Procedures
Preventive Maintenance (PM) Schedule

Table 5: Training dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
Training plan exists for the Lab
Analyst (need to work out
equivalent role)
Training Records Exists for all Lab
Analysts
Training plan exists for the Lab
Analyst and Lab Technologist (need
to work out equivalent roles)
Training Records Exists for all Lab
Analysts and Technologists.
List of All Lab and Quality personnel
with their job title and roles
available.
Training Plan exists for all
personnel and there is appropriate
training modules matching their
roles and responsibilities.
Training Records Exists for all Lab
and Quality personnel
Same as score for 6 +
Every training module/procedure
has a completed competency
assessment.
Element
Score 0 3 6 9
Evidence I am
Looking For
No assessment/qualification
process evident
Qualification process is
documented in their work process
procedures.
There is an
assessment/qualification for each
training module/procedure
Same as score for 3 +
Assessment sheet must include:
Name of Trainee, training module
title, date of assessment, Assessor
name, Questions to prove trainee
understands the training received.
Assessor sign off that the trainee
completed the practical task
sucessfully (only required for
training on practical tasks e.g.
titration, chromotography etc)
Final sign off by trainee + assessor
with date.
Same as score for 6 +
Work process includes rules on re-
assessment/requalification
Element
Score 0 3 6 9
Evidence I am
Looking For
MET training completed by <50% of
the work group.
MET training completed by 50% to
75% of the work group
MET training completed by >75% of
the work group.
MET training completed by 100% of
the work group.
Plan exists with dates for all
personnel to get refresher training
Element
Score 0 3 6 9
Evidence I am
Looking For
No Training Records exist Traning Records exist
Training Record has trainee name,
trainer's name, title of training, and
date when the trainee completed
training.
Same as score for 3 +
Training Records have
- Questons answered.
- Assessor has marked the answers
and indicated a pass or failing mark.
- Assessor sign off that the trainee
completed the practical task
sucessfully (only required for
training on practical tasks e.g.
titration, chromotography etc)
- Final sign off by trainee + assessor
with date.
Same as score for 6 +
Training records show the date all
personnel have to complete
requalification. This time period
meets work process (MET)
requirements.
Training records show
requalification completed by
requalification date
1) Can you show me a list of all the Laboratory and Quality personnel with their job title, roles and responsibilities?
2) Can you show me the training plan required for each Job title?
3) Can you show me the Training record (record of training completion and date + Date of required Requalification) for the Laboratory and Quality
personnel?
4) Do you have an assessment process as part of each of your training modules/procedures?
5) Does the assessment process involve a practical assessment of the Task?
6) Can you show me in your work process (MET) procedures the section on qualification and requalification?
7) From the list of training modules/procedures check that there is an assessment file/sheet for each training module and procedure
8) Pick 3 training modules/procedures and review assessment sheets.
9) Can you show me where the you keep the training assessment records (Records of each individuals sign off for each training
module/procedure)?
10) Look at the Overall Training record and pick 3 records shown as completed and find the completed assessment forms. Review Assessment
against required standard
Training Records
Employee Training Program
Qualification Process
Most Effective Technology (MET) Training

Table 6: AIQ dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No AIQ program Documentation on DQ/IQ/OQ that
has parameters for proper
operation
There's PQ data showing stability
(e.g. check standards run every
week)
Same as 6, but even higher level
plan around qualifying instruments
that are currently not qualified.
Risk-based mechanism to manage
qualifications
Element
Score 0 3 6 9
Evidence I am
Looking For
AIQ process doesn't exist or is not
used
33% of quality-critical instruments
have used AIQ
67% of quality critical instruments
have used AIQ
100% AIQ for quality-critical devices
and evidence that this is standard
as part of the start-up process
Element
Score 0 3 6 9
Evidence I am
Looking For
Hard copy of initial qualification
records for 0-33% of quality-critical
instruments
Same as 0, but >33% Same as 3, but >67% 100% of instruments have a hard
copy of the initial qualitifcation
records, and records demonstrate
that the instrument has consistent
performance
1) Explain how new equipment is acquired and integrated into the lab.
2) Do you collect Design Qualification (DQ), Installation Qualification (IQ) and Operation Qualification (OQ). data? How many instruments do you
do this on? Can you show us?
3) Do you collect PQ data? Can you show us?
4) Do you save the initial qualification data on hard copy? Can you show us?
Analytical Instrument Qualification (AIQ) Program
Implementation of Instrument Qualification Process
Instrument Qualification Records

Table 7: SQC dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No documented SQC procedure
exists
SQC procedure exists and contains
- Calibration requirements and
frequencies
- Check Standard requirements and
frequencies
Same as score for 3 +
SQC procedure contains
- How Control limits are calculated
- SQC rules in use to define a
measurement as out of control
Same as score for 6 +
SQC procedure contains
- Actions to take if a measurement
is out of control
Actions I would expect to see are:
- Investigation of Issue
- Action taken to resolve issue
- Machine taken out of use until
issue is resolved
- All analysis ran since machine was
last within control are rechecked to
determine if results are correct
Element
Score 0 3 6 9
Evidence I am
Looking For
No SQC Records Available SQC Data (Control charts with
anlysis points marked on a chart) is
available in hard copy
SQC data available in electronic
system
- Data entered into electronic
system
- Control charts generated showing
performance
Same as score for 6 +
Current and historical SQC data can
be accessed easily within an
electronic system.
SQC data includes control limits
with automatic indication if any
results are out of control according
to SQC rules.
Action log with records for every
incident where the analysis is out
Element
Score 0 3 6 9
Evidence I am
Looking For
Sample analysis is not recorded in
electronic system.
Or
Sample analysis recorded in
electronic system but no
automated valuation of the result
against the specification occurs
Sample analysis recorded in
electronic system.
Results are automatically valuated
against the specification.
Anything outside of the
specification is highlighted as out
of spec.
Same as score for 3 +
All instrument SQC charts exist
within statistical software (excel
with appropriate statistical rules
coded into the spreadsheets would
count)
SQC rules and limits defined in
software.
Evidence that action has been
taken when instrument is out of
control e.g. action log
Same as score for 6 +
Regular review meetings are taking
place looking at the trends from the
statistical data and actions -
evidence would be review meeting
minutes, action log from meeting
showing changes to process due to
evaluation of data (e.g. column
change frequency increased on
instrument due to higher levels of
failure at end of current life)
Charts exist looking at the
perfomance of the instruments.
Charts could show total
perfromance over time,
performance per analysis over time
etc.
Procedures define prevantative
maintenace requiremnts based on
SQC data
Action log shows instrument taken
down for preventative maintenace
based on SQC data.
Element
Score 0 3 6 9
Evidence I am
Looking For
No measurment Capability studies
carried out and documented
1/3 of all the measurements have a
capability study.
2/3 of all the measurements have a
capability study.
All the measurements have a
capability study.
Measurment Capability Review
plan is in place.
Evidence that Reviews are being
completed. Looking to see that plan
is being acted upon.
1) Can you provide me with a copy of the SQC procedure?
2) Can you provide a list of the analytical equipment and the anlaysis that is run on each piece of equipment?
3) Can you show me where the records of your SQC analysis are kept?
4) Can you show me where the records of your analysis are kept?
5) Can you show me where your measurement capability studies for each analysis are kept?
6) Can you show me your measurement capability review plan?
Measurement Capability
Statistical Quality Control (SQC) Procedure
SQC Records
Data Evaluation

Table 8: SOP dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
Less than 25% of the procedures
meet the following requirements
- Format of the Procedure Template
is matched in procedures reviewed.
- Document control section of the
procedure meets the standards set
out in the document control (MET)
procedure
- Management of change section of
the procedure is completed and
meets standards set out in
management of change procedure
- Procedure is in centralised
location available to all personnel
at every Lab
Between 25% to 50% of the
procedures meet requirements
Between 50% to 75% of the
procedures meet requirements
100% of the procedures meet
requirements
Element
Score 0 3 6 9
Evidence I am
Looking For
Less than 25% of procedures
include Personal Protective
Equipment (PPE), hazards and
sufficient details to identify and
eliminate potential hazards.
Between 25% to 50% of procedures
include Personal Protective
Equipment (PPE), hazards and
sufficient details to identify and
eliminate potential hazards.
Between 50% to 75% of procedures
include Personal Protective
Equipment (PPE), hazards and
sufficient details to identify and
eliminate potential hazards.
100% of procedures include
Personal Protective Equipment
(PPE), hazards and sufficient details
to identify and eliminate potential
hazards.
Element
Score 0 3 6 9
Evidence I am
Looking For
Less than 25% of procedures have:
1) clearly defined operating steps
sufficient for a non-routine user to
complete the task successfully;
2) supporting information such as
specification of apparatus and
reagents; and,
3) cross reference to any related
documents such as ASTM.
Less than 50% of procedures have:
1) clearly defined operating steps
sufficient for a non-routine user to
complete the task successfully;
2) supporting information such as
specification of apparatus and
reagents; and,
3) cross reference to any related
documents such as ASTM.
Less than 75% of procedures have:
1) clearly defined operating steps
sufficient for a non-routine user to
complete the task successfully;
2) supporting information such as
specification of apparatus and
reagents; and,
3) cross reference to any related
documents such as ASTM.
All procedures have:
1) clearly defined operating steps
sufficient for a non-routine user to
complete the task successfully;
2) supporting information such as
specification of apparatus and
reagents; and,
3) cross reference to any related
documents such as ASTM.
Element
Score 0 3 6 9
Evidence I am
Looking For
Procedure Use Policy (PUP)
specifies tasks requiring
procedures and documents
requirements for using routine and
non-routine procedures.
All required procedures exist as
defined by PUP. This includes
procedures for sampling,
performing analyses, calibration,
SQC and equipment operation.
Maintenance instructions may be in
the form of SOP's and/or vendor
manuals.
Data collection process verifies use
of non-routine procedures and
usage data is evaluated over time.
Data collection process verifies
usage of non-routine procedures
and verifies that procedures are
executed correctly.
1) Can you provide me with your document control (MET) procedure?
2) Can you provide me with your management of change procedure?
3) Can you provide me with your electronic Template for procedures?
4) Can you provide me with a list of all your procedures?
5) Can you show me where you store your procedures?
6) Who has access to the procedures?
7) Do you have a record of which procedures meet your MET requirements for a procedure?
8) Select 12 procedures and review them comparing to standards required
9) Take 4 procedures and have user run through the task (check that the steps in the procedure match what the user is doing. Also check that there
is enough information in the procedure to complete the task
Procedure Use Policy
Document Control
Hazard Elimination
Procedure Details

Table 9: UPE Management dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No Investigation process or
No documentation in evidence of
formal investigation process
Formal Investigation process
documented in procedure
Procedure must include
- Definition of what is a Quality
failure/incident.
- Triggers/Rules defining when an
investigation should take place.
Formal Log for all quality
failures/incidents exists
Log must include a
- Description of the failure
- Root cause(s) of the issue
identified
- Action Log
Same as 3 +
Quality failures/incident reports
are generated. These should
include
- Description of the failure
- Root cause(s) of the failure
- Description of what the lab has
done to prevent the failure from re-
occurring
Evidence that reports are shared
between Labs
- Shared location for reports to be
viewed
- Section in Regular Lab Meeting
Schedule to review Incident
Reports
Same as 6 +
Regular Management Review
Meeting exist for quality
failures/incidents.
Meeting Minutes show
- Discussion on any trends
(common failures) that exist that
may need a change in the
management system (process &
procedural issues)
- Action Log with evidence that
management system issues are
being addressed
Same Process exists for reporting
and managing quality near misses
as for quality failures/incidents. A
quality near miss is where you have
had a failure or incident that could
have caused a quality issue but
luckily did not e.g. Issue found
with HPLC column. Retesting of
retained samples with new column
found results to have changed but
are still within specification.
Element
Score 0 3 6 9
Evidence I am
Looking For
No CAPA procedure section or
individual procedure exists
CAPA procedure section or
individual procedure exists
Documentation for the
investigation show and identified
root cause for the issue
Same as 3 +
Corrective action log exists with
evidence that actions are being
completed.
Analyse the incidents/failures
looking for the same problem
occurring repeatedly. If root cause
was identified and fixed then you
should not have the same failure
keep repeating.
Same as 6 +
Regular Management Review
Meeting exist for quality
failures/incidents.
Meeting Minutes show
- Discussion on any trends
(common failures) that exist.
- Action Log with evidence that
action is taken if the fixes put in
place are not addressing the issue.
Element
Score 0 3 6 9
Evidence I am
Looking For
Corrective and Preventive Action
(CAPA) procedure not established
and implemented.
CAPA procedure exists to identify
potential sources of non-
conformities and to define
preventive actions.
Evidence shows that preventive
actions focus on systems rather
than single events.
Effectiveness of preventive actions
is evaluated by summarizing CAPA
data and monitoring for
management system failure trends.
Element
Score 0 3 6 9
Evidence I am
Looking For
Protocol not defined &
documented for handling out of
specification situations.
Specific guidelines are documented
for Retesting & Resampling after
obtaining out of specification
results. Guidelines include
documentation requirements.
Nonconforming test results are
routinely investigated and
documented. Calibration
standards, instrument performance
and sample validity are evaluated.
Evidence indicates that all
nonconforming results are
investigated & that corrective
actions are taken to prevent
reoccurrence. Appropriate
techniques are used (i.e.
notification of Quality Coordinator,
reverse traceability, Root Cause
Investigation (RCI), etc).
Element
Score 0 3 6 9
Evidence I am
Looking For
External nonconformance received
related to testing in your lab
>1 Major internal nonconformance
received related to testing in your
lab.
No more than 1 major internal
nonconformance received related
to testing in your lab.
No nonconformances received
related to testing in your lab
1) Do you have a procedure on how you manage quality failures/incidents and can you provide me with the procedure?
2) Does the procedure document what is a classed as a quality failure/incident?
3) Does the procedure define a process that is to be used to investigate quality failures/incidents
4) Does the investigation process have triggers to initiate an investigation and tools to use for the investigation (Root cause investigation)?
5) Do you have any reports (LER's) giving a summary of the issue, the root cause and preventative actions taken to prevent reoccurrence of the
issue?
6) How are theses reports distributed to analytical/quality personnel (allows shared learnings to help prevent similar incident accross all
locations)?
7) Do you hold any management review meetings looking at quality failures/incidents and their investigations? If yes, please provide me with the
minutes from the last 3 meetings?
8) Do you record Quality near Misses? If so, what is the process for collecting this information?
9) Check if there is a Corrective and Preventative Action section in the Manage Quality Failures/Incident procedure. If not, ask to be provided the
CAPA procedure.
10) Can you show me a summary report showing all the incidents/failures for the last year?
11) Pick 3 incidents/failures and ask for the investigation documents recorded during the analysis.
12) Can you provide me with the corrective action log for quality failures/incidents?
13) Can you show documentation around the non-conforming test results and the documentation of investigation?
Control of Nonconforming Test Results
Nonconformances Detected by Inspection
Investigation Process
Corrective Actions
Preventive Actions

Table 10: EH&S dimension questions and audit evidence
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
Either don't measure or Recordable
Rate > 0.40.
Recordable Rate not more than
0.40.
Recordable Rate not more than
0.30.
Recordable Rate not more than
0.20.
Element
Score 0 3 6 9
Evidence I am
Looking For
Group does not have or require
personal safety goals.
10-30% of empolyees have
personal safety goals.
31-60% of employees have
personal safety goals.
>61% of employees have personal
safety goals.
Element
Score 0 3 6 9
Evidence I am
Looking For
Near misses not recorded Near misses recorded Same as 3+
Goals set for employees regarding
near miss recording
Same as 6+
Data reviewed/analyzed to view
trends and take action.
Evidence: Review meeting,
meeting minutes, trends and
actions
Element
Score 0 3 6 9
Evidence I am
Looking For
LOPC's not identified. LOPC's identified. LOPC's identified and tracked.
Evidence: Tracking tool, updated
recently
LOPC's identified, tracked, and
systems in place to reduce LOPC's.
Evidence: Analysis of LOPC trends
and actions taken and results.
Element
Score 0 3 6 9
Evidence I am
Looking For
No minimum EH&S requirements
exist for the labs.
EH&S lab requirements have been
identified and documented
EH&S lab requirements have been
identified, the lab audited, gaps
identified, and a timeline in place
to close the gaps.
Full compliance with minimum
EH&S lab requirements.
Element
Score 0 3 6 9
Evidence I am
Looking For
Critical Instrument plan not
implemented or critcal instrumnets
not identified.
Critical Instruments identified, but
gaps not identified.
Critical Instruments identified, gaps
identified, and gap closure plans in
place.
Critical Instruments Identified, gaps
identified, and gaps closed with full
compliance, or Critical Instruments
plan is determined to be not
applicable to Lab entity.
Element
Score 0 3 6 9
Evidence I am
Looking For
Life Critical Standards not
implemented.
All Life Critical Standards identified
and Life Critical Standards utilized.
All Life Critical Standards
identified, Life Critical Standards
utilized, and Life Critical Standards
audited with passing results < 90%
of time.
All Life Critical Standards
identified, Life Critical Standards
utilized, and Life Critical Standards
audited with passing results 90% of
time.
1) What is you recordable injury rate? (Generally, a recordable injury or illness under OSHA is one that requires medical treatment beyond first
aid, as well as one that causes death, days away from work, restricted work or transfer to another job, or loss of consciousness. The number of
injuries need to be divided by the total number of work hours performed, and put in the format of [(#injuries)/(200,000 work hours)]
2) Does the group have personal safety goals? What percentage of lab personnel have individual goals that align to safety?
3) Do you record "near-misses" (injuries that have almost occurred, but did not)? Can you show me where they are recorded?
4) Are you analyzing "near-misses" for trends? Can we see an analysis?
5) Do you meet to review "near-misses" regularly? Can we see meeting minutes of the last meeting?
6) Do you identify leaks, breaks, spills, otherwise known as Loss of Primary Containment Incidents(LOPCs)?
7) Do you record LOPCs formally?
8) Do you have a system in place to analyze and reduce the number of LOPCs?
9) Have you defined formal lab EH&S Requirements?
10) Do you assess the labs against these EH&S requirements?
11) Do you track progress against these EH&S requirements?
12) Do you have EH&S Critical Instrumentation? (Fire alarms, leak detection, vapor or fume alarms)?
13) What monitoring do you have in place (inspection, maintenance) for these EH&S Critical Instruments?
14) Are there any gaps? If so, are there plans in place to close the gaps?
15) Do you have Life Critical Standards (how to perform hot work, open lines, do electrical work, perform elevated work, confined space entry)
16) Do you utilize these Life Critical Standards?
17) Do you audit against the Life Critical Standards? If so, how often does the audit pass? (>90% or <90%)
Life Critical Standards
Loss of Primary Containment (LOPC)
Lab EH&S Requirements
EHS Critical Instrumented Systems
Lab Recordable Injury Rate
Personal Safety Goals
Near Miss Program

Table 11: Best Practices dimension questions and audit evidence
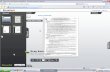
Once the audit is complete, the scoring values on these elements are arrayed into “spider plots” for each individual dimension (see below in Figure 1 for the “enablers” spider plot). The averaged scores for each dimension are also arrayed into a spider plot for a rapid determination of gaps as a function of different bulk areas (Figure 2).
Figure 1: Enablers dimension spider plot showing a score of “3” across all elements
Questions
Element
Score 0 3 6 9
Evidence I am
Looking For
No documented job or role
descriptions in place.
Documented job descriptions in
place for >50% of the work group.
Documented job descriptions in
place for >75% of the work group.
Documented job descriptions in
place for all personnel that define
roles and key job responsibilities.
Job descriptions are reviewed on a
regular basis.
Element
Score 0 3 6 9
Evidence I am
Looking For
Little or no method validation
exists for analyses.
Method validation exists and is
sufficient for 1/3 of the analyses.
Method validation exists and is
sufficient for 2/3 of the analyses.
Existing method validation
sufficient for the analyses. New
methods are validated as per the
EIAR process/procedure
1) Can you show us your method validation procedure? How many methods have gone through this process?
2) Can you show your job roles and descriptions? Do you review these?
Role and Job Descriptions
Method Validation

Figure 2: Dimension spider plot showing a score of “3” across all dimensions
These dimensional audit areas are analyzed by the lab leadership to determine a multi-year and multi-generational plan that will get the lab closer to an idealized final result. The leadership determines what focus areas and specific remedies need to be executed and when. This tool can be used among different labs or re-administered to the same group after a year or more to see if there’s been quantitative progress.
Change Management Overview: It was understood, through conversations with EIAR leadership during the project scoping
process, that “Lab Unification” was a key medium-term goal. Successful integration of
procedures and processes requires skillful change management, with consideration for the
technical and organizational structure of EIAR.
This project was designed to illuminate both technical and non-technical areas of opportunity.
During the process of addressing these opportunities, or said another way “closing the gaps”,
can be very helpful to standardize lab operating procedures and organizational culture.
Organizational change and more specifically organizational culture change is not something
that is achievable rapidly without significant disruption to operations. It will take a concerted

and prioritized effort by lab leadership over many months. This team recommends using the
audit as a tool as a guide to structure the change program that will need to be established.
Open and transparent communication about the lab unification effort, as well as dialogue with
key stakeholders from various labs will be critical for successful implementation. Different labs,
due to the uniqueness of the individual researchers and staff members, may require different
approaches. As with any large organization, sub-groups and sub-cultures surely exist within
EIAR, and the approach for specific labs may need to be modified accordingly. However, it is
recommended that the core of the change program be an understood vision of the future,
common goals, and clear description of the ideal state that is defined by EIAR leadership,
through consultation with key organizational stakeholders. The audit tool is designed to
facilitate the discussion of what a world-class lab management program consists of. It is role of
EIAR leadership, however, to refine these criteria through the vision and mission of EIAR to
communicate a vision for the future that is best understood by EIAR staff. The concept of
engaged employees committed to continuous self-improvement is foundational to the success
of this program.
Establishment of an Interdisciplinary Executive Team:
It is our recommendation that EIAR form an Executive Team comprised of representatives from
various disciplines to consider the following in addition to the audit tool descriptors of ideal lab
operating conditions:
Consider how EIAR’s Vision and Mission can be a constructive frame through which to
prioritize lab improvements.
Implementation timeline: Is this a 5-year project? A 3-year project?
Consider the resources available to the change effort. How much change can the
organization tolerate? How can you continue to remain effective while also improving?
Consider data collection: Data will be collected (the lab audit is one source of data) that
will identify areas of opportunity for EIAR. What other data are necessary? (employee
surveys, discussions, feedback collection)

Summary of Audit Findings: What follows are two separate prioritizations for action. First, priority is measured by element. This element-by-element prioritization provides a detailed view of which elements should receive the highest priority. More often, however, it is useful to consider which dimensions have the highest priority overall. Often, a thoughtful project plan will consider both aspects in order to generate a comprehensive multi-generational plan. These priority orders are the assessment generated by the Dow team, but EIAR may wish to consider how best to prioritize based upon EIAR-specific needs and capabilities. The Audit findings for each element are listed below along with the prioritization score that highlights the elements in order of most benefit for least amount of effort. The Benefit and effort scoring were accomplished using a scoring system of 1, 3 and 9 (1 being Low, 3 being medium and 9 being high benefit and effort). The priority is worked out by the calculation below:
Dimension Element
Element Score
for EIAR Lab
Benefit of Improving to Next Level(s)
Effort to Implement
Priority to Improve
EH&S Goals and Perf. Tracking
0 9 1 9
EH&S Environmental Excellence
0 9 1 9
EH&S Lab EH&S Requirements
0 9 1 9
Calibration Calibration/Verify Instructions
6 9 1 9
Calibration Calibration Records/Reports
0 9 1 9
Preventive Maintenance
Maintenance Strategy
0 9 1 9
SQC SQC Records 0 9 1 9
SOP Hazard Elimination 0 9 1 9
Enablers MET Implementation 0 9 3 3
Processes MOC Process 3 9 3 3
Training MET Training 0 9 3 3
SQC SQC Procedure 0 9 3 3
Preventive Maintenance
PM Schedule 0 3 1 3

SQC Data Evaluation 0 9 9 1
Enablers Client Satisfaction 0 3 3 1
Calibration Calibration/Check Schedule
6 3 3 1
Preventive Maintenance
PM Records 3 3 3 1
Training Qualification Process 3 3 3 1
Training Training Records 3 3 3 1
AIQ Qualification Program
3 3 3 1
Unplanned Events Management
Investigation Process 3 3 3 1
Unplanned Events Management
Preventive Actions 3 3 3 1
Processes Service Level Agreements
0 1 1 1
Processes Sample Plan 6 1 1 1
Preventive Maintenance
PM Procedures 6 1 1 1
Processes Sample & Data Management
3 3 9 0.333333333
Processes Sample and Work Load
3 3 9 0.333333333
Unplanned Events Management
Nonconforming Results
6 3 9 0.333333333
Processes Non-Routine Request Process
3 1 3 0.333333333
Processes Lab Inventory & Capital Plan
6 1 3 0.333333333
Calibration Vendor Calibrations 6 1 3 0.333333333
AIQ Implementation of AIQ Program
0 1 3 0.333333333
AIQ Qualification Records 6 1 3 0.333333333
SQC Measurement Capability
6 1 3 0.333333333
Unplanned Events Management
Corrective Actions 6 1 3 0.333333333

Unplanned Events Management
Nonconformances by Inspection
3 1 3 0.333333333
Enablers People Success 0 1 9 0.111111111
Calibration Standards and Reagents
6 1 9 0.111111111
Preventive Maintenance
Parts Inventory 9 1 9 0.111111111
Training Employee Training Program
9 1 9 0.111111111
SOP Document Control 9 1 9 0.111111111
SOP Procedure Details 9 1 9 0.111111111
SOP Procedure Use 0 1 9 0.111111111

Lab Audit Details
When determining what to work on first it is usually best to complete the tasks that require the least effort with the biggest benefit (A common US/UK expression for these items is “Low hanging fruit”). You should also look at the items that have a high benefit but require more significant levels of effort as these may be very important to for your organization. In some cases these items may need to be started earlier than their priority suggests because they are very important and are only lower due to the time and effort. The low hanging fruit to work on first are (general prioritization by dimension):
Dimension Element Actions Required
EH&S Goals and Perf. Tracking
EH&S Goals should be established
EH&S Environmental Excellence
EIAR should consult with other local agencies to establish environmental plans that are meaningful and achievable.
EH&S Lab EH&S Requirements
Safety: It is the responsibility of EIAR to establish a safety culture. Some priority areas to address include, but are not limited to the following standard safety measures:
Personal Protective Equipment (hand and eye protection)
Operational fume hoods
Safety eye-wash stations and safety showers
Proper storage of laboratory chemicals and reagents
SQC SQC Records
Create a Check Standard(s) for each calibration with known quantities of everything the machine is required to measure. Run Check standard at a set frequency and record data on an excel sheet. Plot this data in graphs in excel. Once sufficient information is gathered then Develop Control Limits (+/- 3 sigma) and plot these on the graph.

Dimension Element Actions Required
SOP Hazard Elimination
Each Lab procedure is to have a Hazards section added with all the potential Hazards associated with the task being carried out in the procedure. Each Lab procedure will outline the Personal Protective Equipment (PPE) required to carry out the task being carried out in the procedure.
Calibration Calibration/Verify Instructions
Instructions added to each calibration procedure on what to do if the calibration fails. These instructions should include:
- Where the failure should be documented and How the failure should be documented
- Whom (May be a role rather than a person e.g. Lab Manager) the failure should be communicated.
- The information that should be communicated - What action to take to confirm that analysis
completed since last calibration is correct. - Under what conditions the instrument should
be taken out of service (e.g. how many attempts at re-calibration)
Calibration Calibration Records/Reports
Calibration record created electronically (could use excel) for all calibrations carried out. Records to include
- Date and Time calibration is carried - Analyst who carried out the calibration - Equipment the calibration was carried out on - Analytical results for calibration are documented - Result of Calibration are documented (e.g. Pass
or Fail)
Preventive Maintenance
Maintenance Strategy
A maintenance strategy should be established that is achievable and value-oriented.
The Elements that need to be worked on first even though they require more effort are:
Dimension Element Actions Required

Dimension Element Actions Required
Enablers MET Implementation
The Implementation of a standardised work process (MET) across all labs will take quite a long time to fully design and implement. This step is critical to ensuring that you function as one organisation and that you can rely on getting the same result for the analysis no matter which laboratory the sample is tested. First a set of MET requirements/procedures need to be written by the senior leadership on the requirements/work process they want all the labs to follow. This should be a high level document aligning with your strategy in each of these areas. I would expect to see MET requirements/procedures for most of the sections in the Audit
- EHS - Management of Change Process - Sample and Data Management Process
o Sample/plan and None routine analysis o Results recording and analysis
- Calibration and Statistical Quality Control Process
- Preventative Maintenance Process - Instrument Qualification Process - Unplanned Events Management - Standard Operating Procedures - Training Process

Dimension Element Actions Required
SQC SQC Procedure
Create SQC procedure for Lab. Lab procedure must comply with EIAR’s MET requirements for statistical quality control. The procedure should contain
- Frequency of testing for each check standard - Frequency of calibration for each analysis (I
advise starting with a set frequency for calibration and use the analytical data from the check standards over time to determine if the frequency needs to be extended or shortened. Eventually moving to full statistical control to determine when to calibrate the machine)
- Procedure to contain statistical rules determining when the analysis is out of control (you may want to start with a simple rule +/- 3 sigma and add in more advanced rules as you have the ability. Statistical software or more advanced excel sheets with code required to run more advanced rules)
- Actions to take when the analysis is out of control. This should include
o when to re-calibrate o when to take the machine down for
further analysis of the problem o when to remove the machine from
service o The analysis that needs to be re-tested.
As you are unsure when the machine went out of control since the last check, all analysis since that time may be incorrect. Once machine has been fixed then the suspect analysis needs to be re-checked to confirm results are correct.
SQC Data Evaluation
An electronic Laboratory Information Management System is required to record and evaluate results. Below are some open source (free) and proprietary source LIMS systems.

Free and open-source LIMS software
LIMS software
Language base
License Other info Website
LabKey Server
Java Apache
Software License
primarily a web-based data management platform
http://www.labkey.org
MISO Java GPLv3
Web-based information management system for next-generation sequencing (NGS) experiments, developed at The Genome Analysis Centre
www.tgac.ac.uk
Bika LIMS
Python - Zope - Plone (linux
based)
GNU AGPLv3
Web-based Lab Information Laboratory Management System for clinical, water, ambiental, chemical, microbiological and others analytical services.
http://www.bikalabs.com/
Proprietary LIMS software
LIMS System Website
Accelrys LIMS from Accelrys www.accelrys.com
ApolloLIMS from Common Cents Systems, Inc. www.apollolims.com
Biotracker from Ocimum Bio Solutions www.ocimumbio.com
Biotracker Lite from Ocimum Bio Solutions www.ocimumbio.com
CaliberLIMS from Caliber Technologies Pvt. Ltd. www.caliberindia.com
CCLAS from Ventyx, an ABB company, formerly Mincom (company)
www.ventyx.com
Clarity LIMS from GenoLogics Life Sciences www.genologics.com
CloudLIMS from CloudLIMS www.cloudlims.com
Darwin from Thermo Fisher Scientific www.thermofisher.com
ELab from LabLynx www.lablynx.com
Element LIMS from Promium www.promium.com
Exemplar Biomarker Discovery from Sapio Sciences www.sapiosciences.com
Exemplar Dx LIMS from Sapio Sciences www.sapiosciences.com
Exemplar Research LIMS from Sapio Sciences www.sapiosciences.com
Galileo from Thermo Fisher Scientific www.thermofisher.com
LABbase from Analytik Jena www.analytik-jena.com
LABVANTAGE from LABVANTAGE Solutions www.labvantage.com
Labware from LabWare www.labware.com/en
LABWORKS from PerkinElmer www.perkinelmer.com
Labway-LIMS from Ambidata Digital Innovation Solutions & Consulting
www.ambidata.pt

LDMS from Frontier Science and Technology Research Foundation
www.fstrf.org
Matrix Gemini from Autoscribe www.autoscribeinformatics.com
Nautilus from Thermo Fisher Scientific www.thermofisher.com
ProlabQ from Open-Co
www.openco.it/index.php?option=com_k2&view=item&id=9:prolabq-lims&Itemid=39&lang=en
readyLIMS from Analytik Jena www.analytik-jena.com
Result Point from Accelerated Technology Laboratories, Inc www.atlab.com
SampleManager from Thermo Fisher Scientific www.thermofisher.com
Sample Master from Accelerated Technology Laboratories, Inc www.atlab.com
SIMATIC IT Unilab from Siemens www.Siemens.com
STARLIMS from STARLIMS Corporation www.starlims.com
TITAN from Accelerated Technology Laboratories, Inc www.atlab.com
Watson from Thermo Fisher Scientific www.thermofisher.com
webLIMS from LabLynx www.lablynx.com
WinLIMS from QSI Corporation N www.qsius.com
NuGenesis from Waters Corporation www.Waters.com
Secondary Elements to work on
Dimension Element Actions Required
Processes MOC Process
Develop management of change process and procedure. This procedure will outline the different triggering criteria that would require the initiation of MOC as well as standardize what type of communication needs to occur within the organization. For example, changing a procedure requires lab leadership approval and distribution to all the lab personnel.
Training MET Training Train all personnel in the MET requirements/procedures
Enablers MET Implementation
Each Lab to review its procedures to ensure that they all comply with the MET requirements/procedures. Individual Lab procedures updated to conform to the new MET requirements/procedures.

Dimension Element Actions Required
Preventive Maintenance
PM Schedule
Create preventative maintenance schedule for all instruments. A place to start is manufacturer’s recommendations as a function of use. Secondly, the lab can use the SQC data that will ideally be acquired from the recommendations above. These data will provide information about how often the equipment needs to be preventively maintained in order to provide the required performance. These data can be leveraged among multiple labs.
SQC SQC Records
Use statistical software or advanced excel sheets to record your SQC data having the SQC rules built in so that if the result is outside of your rules then it is easily identified when the result is entered. Create Action log for each control chart. This action log should include
- Date and Time of Analysis - Analyst who carried out the analysis - Issue with analysis e.g. control outside of upper
control limit. - Action taken e.g. equipment re-calibrated and
checked. Samples since last check re-analysed and all results are correct.

Prioritization and Multi-Generational Plan
The Audit Tool is designed to highlight gaps, but not all gaps can be addressed simultaneously, nor should they be addressed with the same level of emphasis. It is therefore critical to consider both the priority management gives to certain sub-elements, as well as when the issues will be addressed. These two elements should be managed by the executive leadership team, and key concepts that were reviewed during the project are described below. For system improvement, there is no inherently right or wrong answer for what to work on first. The lab audit results may show some obvious high-priority areas to improve, but there may be a large “middle ground” of neither high nor low priority items that need to be addressed. In this case, EIAR needs to consider the benefits that would be gained from each item and the resources needed to pursue them. Step 1: Develop List of “Improvement Projects”: The audit findings will highlight the gaps to the “ideal state”, but projects will be necessary to address these gaps. These projects will have different benefits, and will be viewed by different stakeholders differently. As such, stakeholder management is a critical step to successful change. Step 2: Determine Benefit by Stakeholder: Different stakeholders in EIAR will have different priorities and will be benefited by change differently. The executive team should be the clearinghouse for high-level strategic concepts and set the direction, taking into account EIAR’s larger goals. For agreement on path forward, it may be helpful to consider benefits by stakeholder group or by element of EIAR’s mission/vision. One way to generate an organization-wide benefit ranking is to use a Pugh Matrix, with 1, 3, and 9 ranking scale. Each stakeholder should determine the benefits they or their group would receive. The SUM of the benefits should be then used to prioritize. Example Pugh Matrix:
Stake-holder 1 Stake-holder 2 Stake-holder 3 SUM
Project 1 1 3 3 7 (lowest priority)
Project 2 1 9 3 13

Project 3 3 1 9 13
Project 4
3 9 9 21 (highest priority)
Project 5 9 1 1 11
Step 3: Determine “must-do” improvements that will proceed regardless of effort required.
Health and Safety projects fall into this category.
Step 4: Plot Improvement Projects as follows:
Multi-Generation Plan:
Multi-Generation Plan (MGP): The improvements, once plotted, can be used to develop a Multi-Generation Plan (MGP). The MGP is a guide for what to work on first, next and last. It should be re-evaluated periodically as you progress, to ensure that priorities have not shifted and that resources needed for projects are re-evaluated. Logically, projects that yield the highest benefit and the lowest required effort (denoted in the shaded area 1, above) should receive attention first. Also important to note are projects that yield very high benefit, but may take a considerable amount of time. These projects may need to begin earlier (receive higher priority) than the MGP might suggest due to the high magnitude of the benefit, or the strategic direction of the Executive Team.

Key concepts for the Interdisciplinary Executive team to consider:
Link the changes to the strategic plan. Ensure that you can explain why you are initiating
the change, and be ready to talk about it.
Determine success criteria: What does success look like?
Allocate resources effectively. Change requires effort above and beyond core job
responsibilities.
Develop training on the change, and allow people the time to ask questions and learn.
Communicate to the organization the strategy and the changes that support it.
Buy-in is critical. One way to increase buy-in is to have the change designed by
empowered sub-teams comprised of those impacted by the change. For instance: a
change that will affect all labs should not be comprised of team members from only 1 or 2
labs.
Give sub-teams a goal (the improvement), the definition of success, resources, and a
timeline. Make the team responsible for refining the improvement and implementing it.
Guide the sub-teams on how to communicate, which stakeholders to involve, and how
often to report back.
Develop a rewards structure for success. Recognize the teams and their results
appropriately.
Be patient – change takes time!
Executing and Implementing Change – Making it “stick”:
Once the improvement has been defined, and is ready for implementation, communication and
training become critical to success. Everyone who is impacted by a change should be informed
of it, and given the opportunity to ask questions or state their opinion, even if they do not have
decision-making authority. Structural changes may be necessary (procedure change,
equipment change) and should be well documented and completely thought through. Follow-
up should occur periodically to ensure that the change has occurred (another audit, for
instance) and improvement should be measured and celebrated.

As stated initially, this should be an ongoing effort, and one which should not have a definitive
end-date. All organizations can improve, and the excellent organization is constantly looking
for ways to improve itself. Evaluate the improvements. What has gone well? What has not
gone well? What has the executive team learned? What has the organization learned?
More Resources:
There is considerable management literature on managing change, improving organizations and
creating learning organizations. This is intended to be a high-level framework for change
management, but is not comprehensive. The executive team may find specific challenges that
are not addressed here, and may find solutions in management journals and books on the
subject. A book that may provide helpful approaches and was discussed during our in-person
visit is:
Stavros, J. M., & Hinrichs, G. (2011). The Thin Book Of® SOAR: Building Strengths-
Based Strategy. Thin Book Publishing.
Related Documents