DOI: 10.1542/neo.10-7-e351 2009;10;e351 Neoreviews Jamie B. Warren and JoDee M. Anderson Core Concepts : Respiratory Distress Syndrome http://neoreviews.aappublications.org/content/10/7/e351 located on the World Wide Web at: The online version of this article, along with updated information and services, is . ISSN: 60007. Copyright © 2009 by the American Academy of Pediatrics. All rights reserved. Print the American Academy of Pediatrics, 141 Northwest Point Boulevard, Elk Grove Village, Illinois, it has been published continuously since . Neoreviews is owned, published, and trademarked by Neoreviews is the official journal of the American Academy of Pediatrics. A monthly publication, at Health Sciences Library State Univ Of New York on August 13, 2012 http://neoreviews.aappublications.org/ Downloaded from

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript
DOI: 10.1542/neo.10-7-e3512009;10;e351Neoreviews
Jamie B. Warren and JoDee M. AndersonCore Concepts : Respiratory Distress Syndrome
http://neoreviews.aappublications.org/content/10/7/e351located on the World Wide Web at:
The online version of this article, along with updated information and services, is
. ISSN:60007. Copyright © 2009 by the American Academy of Pediatrics. All rights reserved. Print
the American Academy of Pediatrics, 141 Northwest Point Boulevard, Elk Grove Village, Illinois,it has been published continuously since . Neoreviews is owned, published, and trademarked by Neoreviews is the official journal of the American Academy of Pediatrics. A monthly publication,
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
Core Concepts:Respiratory Distress SyndromeJamie B. Warren, MD,*
JoDee M. Anderson, MD,
MSEd(c)*
Author Disclosure
Drs Warren and
Anderson have
disclosed no financial
relationships relevant
to this article. This
commentary does not
include a discussion
of an unapproved/
investigative use of a
commercial
product/device.
Objectives After completing this article, readers should be able to:
1. Define respiratory distress syndrome (RDS).2. Discuss the epidemiology, pathophysiology, and diagnosis of RDS.3. Create a differential diagnosis for respiratory distress in the neonate.4. Describe the proven treatments for RDS, with particular attention to antenatal steroids
and surfactant replacement therapy (SRT), their benefits and possible complications.5. Discuss ventilation strategies that can be used in the infant who has RDS.6. Describe long-term complications of RDS and its treatments.
AbstractRespiratory distress syndrome (RDS) is seen primarily in the preterm neonate and isdue mostly to pulmonary surfactant deficiency. Lung atelectasis leads to ventilation-perfusion mismatching, hypoxia, and eventual respiratory failure in the untreatedinfant who has RDS. RDS is diagnosed by physical findings consistent with respiratorydistress and characteristic radiographic findings. Treatment of RDS begins antenatallywith the administration of maternal steroids to women at risk of preterm deliverybetween 24 and 34 weeks’ gestation. The use of repeat doses of antenatal steroids isunder investigation but is currently not recommended outside of randomized, con-trolled trials. SRT has been approved for use since 1990 and has been successful indecreasing rates of RDS. Natural surfactant is currently recommended for use, butsynthetic surfactant that contains proteins to mimic surfactant proteins is beinginvestigated. In general, prophylactic use of surfactant is recommended over rescuetreatment in infants at high risk for developing RDS, but the determination of whichinfants are at high risk for developing RDS remains a clinical one. The push toward useof less invasive ventilation strategies in the treatment of RDS has led to several trials ofnasal continuous positive airway pressure (nCPAP). Results of the SUPPORT trial arepending, but the COIN trial has concluded that nCPAP use in infants who have RDSis not detrimental. Inhaled nitric oxide for RDS still requires investigation on safetyand efficacy. Several other treatments have been studied, but as of yet, only inositoladministration shows promise in the treatment of RDS. Several complications of therecommended treatments for RDS have been identified, but the benefits far outweighthe risks. Finally, there remains a need for long-term follow-up studies on preterminfants treated for RDS to assess neurodevelopmental outcomes.
DefinitionRDS, formerly known as hyaline membrane disease, occurs in incompletely developedlungs and is, therefore, a disease of prematurity. Immature lungs are functionally deficientin mature surfactant. (1) The absence of surfactant in the liquid film lining of alveoli causesan increase in surface tension and alveolar collapse. (2) If not treated, such atelectasis causesan increased work of breathing, intrapulmonary shunting, ventilation-perfusion mismatch,hypoxia, and eventual respiratory failure. (1)
*Oregon Health and Science University, Portland, Ore.
core concepts
NeoReviews Vol.10 No.7 July 2009 e351
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
EpidemiologyRDS is seen almost exclusively in preterm infants, beforethe lungs begin to manufacture adequate amounts ofsurfactant. (2) In fact, the risk of RDS decreases withincreasing gestational age: 60% of babies born at fewerthan 28 weeks’ gestation, 30% of babies born between28 and 34 weeks’ gestation, and fewer than 5% of babiesborn after 34 weeks’ gestation develop RDS. (3) Otherfactors that increase the risk of RDS include male sex,maternal gestational diabetes, perinatal asphyxia, hypo-thermia, and multiple gestations. (4) Antenatal steroidsand prolonged rupture of membranes decrease the risk ofRDS. (5) With the advent of therapies for RDS, includ-ing antenatal steroids and SRT, mortality from RDS hasdecreased from nearly 100% to less than 10% in recentyears. (6)
Differential DiagnosisThe differential diagnosis of respiratory distress in thenewborn encompasses upper respiratory obstruction,pulmonary disease, cardiac disease, thoracic causes, met-abolic disorders, diaphragmatic causes, neuromusculardiseases, infectious causes, hemolytic/vascular causes,and miscellaneous causes (Table 1). (7)(8)
PathophysiologyNormal Lung Development
The period of viability begins at around 23 weeks’ ges-tation, when the fetal lung begins to transition from thecanalicular to the saccular stage of development (Table2). (9) During the saccular stage, peripheral airways
enlarge and distal airways begin to dilate while their wallsbegin to thin. (10) Type II pneumocytes, the cells re-sponsible for surfactant production, are present and ma-turing. (10) Although gas exchange is possible duringthis stage, total surface area for gas exchange is low anddiffusion distance for gas exchange is high in relation tobody weight and metabolic rate. (9) Secondary septa-tion, or alveolarization, begins at about 32 weeks’ gesta-tion. (9) During this phase, alveoli form and mature andalveolar walls thin. (10) All cell types proliferate duringthis phase, including type II pneumocytes. (10) Theoverall result is a maturing lung with a larger surface areaand a minimal diffusion distance for gas exchange. (10)
Surfactant Composition and Life CycleSurfactant is a mixture of phospholipids and proteins. (2)The most abundant surface-active phospholipid in ma-ture lungs is phosphatidylcholine. (11) Phosphatidylcho-line forms a monolayer on the liquid film lining of thealveolus, lowering the surface tension of that film. (2) Inaddition to phospholipids, surfactant contains four majorproteins: surfactant proteins (SPs) A, B, C, and D (Table3). (11) SP-A helps to regulate surfactant secretion anduptake; SP-B and SP-C facilitate adsorption and spread-ing of phospholipids on the liquid film lining of thealveoli. (2) SP-D may play a role in surfactant reuptakeand recycling. (5)
Pulmonary surfactant is manufactured in the Golgiapparatus and stored in lamellar bodies of type II pneu-mocytes. (5) Once secreted by the lamellar bodies intothe extracellular space, surfactant is organized into tubu-lar myelin, adsorbed into the air-water interface, andformed into a lipid monolayer. (5)(6) The surface-activeproperties of the lipid monolayer decrease the surfacetension of the air-water interface and prevent alveolarcollapse. (6) The majority of surfactant constituents arebelieved to be recycled, either through reuptake by typeII pneumocytes or by alveolar macrophages. (9)
RDSAn infant born before the alveolarization stage of lungdevelopment has underdevelopment of alveolar sacsand difficulty with oxygenation and ventilation. (9) Sim-ilarly, an infant born before this stage of lung develop-ment experiences a delay in production and secretion offunctional surfactant. (9) Such surfactant deficiency isthe major reason for poor lung function in the pretermneonate (Table 4). (2)
Although the preterm neonate does produce a smallamount of surfactant, this surfactant contains lowamounts of phospholipids and SPs. (9) It is estimated
Abbreviations
AT: antithrombinBPD: bronchopulmonary dysplasiaCLD: chronic lung diseaseFRC: functional residual capacityGBS: group B StreptococcusiNO: inhaled nitric oxideIVH: intraventricular hemorrhagenCPAP: nasal continuous positive airway pressureNIH: National Institutes of HealthNIMV: nasal intermittent mandatory ventilationPIE: pulmonary interstitial emphysemaPVL: periventricular leukomalaciaRDS: respiratory distress syndromeSP: surfactant proteinSRT: surfactant replacement therapy
core concepts respiratory distress syndrome
e352 NeoReviews Vol.10 No.7 July 2009
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
that infants who have RDS have surfactant pools ofless than 10 mg/kg compared with pools of up to100 mg/kg in term infants. Such surfactant deficiencynecessitates increased work of breathing to distend alve-oli, which the preterm neonate may not be able toprovide. (2) Diffuse atelectasis ensues and leads to anoverall decrease in functional residual capacity (FRC) ofthe lungs. (2) If an infant is allowed to breathe from an
inadequate FRC, lung injury canoccur. (9) Lung injury leadsto protein exudation and edema,which can inactivate surfactant fur-ther. The acidosis and hypoxia thatresults from atelectasis and lung in-jury further interferes with surfac-tant production. The combinationof these events leads to respiratoryfailure.
DiagnosisClinical Evaluation
RDS presents at the time of or soonafter birth, and symptoms worsenover time. (2) Clinical symptomsof RDS are the same as those ofany other respiratory distress:tachypnea, nasal flaring, chest wallretractions, expiratory grunting,and central cyanosis. (2) In the ex-tremely preterm infant, the onlyclinical symptom of RDS may beapnea. (2) It is important to re-member that some infants whohave RDS exhibit all of these symp-toms, and others may show none.
An accurate history is importantin diagnosing RDS. As stated, RDSis more prevalent in earlier gesta-tional ages, so an accurate estima-tion of gestational age is necessary.Other historical factors must bediscerned, such as antenatal steroidtherapy; maternal history of gesta-tional diabetes; course of labor,including prolonged rupture ofmembranes, maternal fever, groupB Streptococcus (GBS) status andantibiotic therapy; method of deliv-ery; and need for resuscitation.
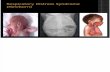
Diagnostic StudiesAlong with the history and physical examination, a chestradiograph is needed for the diagnosis of RDS. Thetypical chest radiograph shows diffuse atelectasis and theclassic “ground glass” appearance of the lung fields (Fig-ure). (2) Air bronchograms, which are air-filled bronchisuperimposed on the relatively airless parenchyma of thelung tissue, also are seen commonly on chest radiograph.(2) Importantly, the appearance of GBS pneumonia on
Table 1. Differential Diagnosis of RespiratoryDistress in the NewbornUpper Airway Obstruction
Choanal atresia, nasal stenosis, Pierre Robin sequence, laryngeal stenosis or atresia,hemangioma, vocal cord paralysis, vascular rings, tracheobronchial stenosis,masses, cleft palate, nasal stuffiness
Pulmonary Diseases
Respiratory distress syndrome, retained fetal lung liquid syndrome (transienttachypnea of the newborn), aspiration (including meconium aspirationsyndrome), pneumonia, pneumothorax, pneumomediastinum, primary pulmonaryhypertension, tracheoesophageal fistula, pulmonary hemorrhage, pulmonaryhypoplasia, pulmonary agenesis, cystic disease, pleural effusion, chylothorax,neoplasm, bronchopulmonary sequestration, pulmonary arteriovenousmalformation, pulmonary interstitial emphysema, pulmonary edema, congenitalalveolar proteinosis, congenital lobar emphysema
Cardiac Diseases
Cyanotic congenital heart disease, acyanotic congenital heart disease, arrhythmia,increased intravascular volume, high output failure, pneumopericardium,cardiomyopathy
Thoracic Causes
Chest wall deformity, mass
Metabolic Disorders
Hypoglycemia, infant of a diabetic mother, inborn errors of metabolism
Diaphragmatic Causes
Hernia, paralysis
Neuromuscular Diseases
Central nervous system damage (birth trauma, hemorrhage), medication (maternalsedation, narcotic withdrawal), muscular disease (myasthenia gravis),intraventricular hemorrhage, meningitis, hypoxic-ischemic encephalopathy,seizure disorder, obstructed hydrocephalus, infantile botulism, spinal cord injury
Infectious Causes
Sepsis, pneumonia (especially group B Streptococcus)
Hemolytic/vascular Causes
Anemia, polycythemia, abnormal hemoglobin
Miscellaneous Causes
Asphyxia, acidosis, hypo/hyperthermia, hypo/hypernatremia
core concepts respiratory distress syndrome
NeoReviews Vol.10 No.7 July 2009 e353
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
chest radiograph can be identical to that of RDS. (12)Empiric antibiotics to address GBS infection should bestarted until such disease is ruled out. Arterial blood gasmeasurements show hypercarbia and hypoxia and even-tually, in the unsupported infant, metabolic acidosis. (2)In all, a preterm infant must have clinical signs of respi-ratory distress and a classic chest radiograph to be diag-nosed with RDS. (2)
ManagementAntenatal Steroids
Antenatal steroid administration to women at high risk ofpreterm delivery prior to 34 weeks’ gestation has beenstandard of care since the 1994 National Institutes ofHealth (NIH) Consensus Conference. (13) A Cochranereview by Roberts and Dalziel from 2006 confirmed thebenefits of antenatal steroids, which include decreases inneonatal death, intraventricular hemorrhage (IVH), andRDS. (1) Antenatal steroids are believed to decrease theincidence of RDS by accelerating maturation of the fetallung. (13)
Early studies on the use of antenatal steroids did notinclude data on babies who were delivered before
28 weeks’ gestation, so there was a question of whetherantenatal steroids would be beneficial in this age group.The Roberts and Dalziel review shows that when steroidsare administered initially at 26 weeks’ gestation, there isa decreased incidence of RDS that is not seen if steroidsare administered before 26 weeks’ gestation. (1) How-ever, the incidence of IVH still may be reduced if steroidsare administered at fewer than 26 weeks’ gestation. (1)Therefore, because of the apparent benefit to preterminfants in terms of decreased IVH, antenatal corticoste-roid administration is recommended for preterm infantsstarting at 24 weeks’ gestation. (13)
Both betamethasone and dexamethasone have beenstudied and found to be more effective than placebo, butthese steroids have not been examined head-to-head.(13) The Roberts and Dalziel review suggests that beta-methasone may cause a larger reduction in RDS thandexamethasone. (1) Baud and colleagues (14) found thatantenatal exposure to betamethasone, but not dexa-methasone, is associated with a decreased risk of periven-tricular leukomalacia (PVL) in preterm infants, but thereis no difference in the incidence of cerebral palsy. (1)With this limited evidence, two doses of betamethasoneadministered 24 hours apart is currently the recom-mended steroid for antenatal use. (13)
Antenatal steroid administration has been shown tobe beneficial if provided fewer than 24 hours before
Table 2. Normal Lung Development (10)
Phase Embryonic Pseudoglandular Canalicular Saccular Alveolar
Gestation(weeks)
�0 to 7 �7 to 17 �17 to 27 �28 to 36 �36�
Structures Trachea andbronchi
Conducting airwaysand terminalbronchioles
Respiratory bronchioles,alveolar ducts,primitive alveoli
Enlarged peripheralairways, thinnedalveolar walls
Definitive alveoli
Type IIPneumocytes
Absent Immature;undifferentiated
Immature;differentiated
Developing laminarbodies
Mature
Table 3. Surfactant Proteins andTheir Functions (5)
SurfactantProteins Functions
SP-A Part of the host innate immune defenseFacilitates the formation of tubular
myelinRegulates surfactant secretion and uptake
SP-B Promotes adsorption and spreading ofpulmonary surfactant
SP-C Promotes adsorption and spreading ofpulmonary surfactant
SP-D Part of the host innate immune defenseMay play a role in pulmonary surfactant
reuptake and recycling
Table 4. Results of SurfactantDeficiency (2)
1. Decreased lung compliance2. Unstable alveoli3. Decreased functional residual capacity4. Hypoxia (from shunting of blood through atelectatic
portions of the lung)5. Increased work of breathing6. Lung edema (exudation of fluid and serum proteins)
core concepts respiratory distress syndrome
e354 NeoReviews Vol.10 No.7 July 2009
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
delivery. Therefore, steroid administration is recom-mended before delivery of preterm infants 24 to34 weeks’ gestation unless delivery is imminent. (13)Furthermore, a reduction in RDS has been seen in infantsborn up to 7 days after the first dose of antenatal steroidswas administered. (1) No benefit is seen in infants whoreceive the first dose of steroids more than 7 days beforebirth. (1)
Because antenatal steroids seem to be of benefit onlywhen administered from just before birth to 7 daysbefore delivery, the utility of repeated antenatal steroiddosing has been studied. The latest Cochrane review onthe subject, conducted by Crowther and Harding in2007, suggests that repeat doses of prenatal steroids doreduce the incidence and severity of neonatal lung dis-ease in the first few postnatal weeks. (15) They recom-mend repeat doses of corticosteroids in women at risk forpreterm birth when the first course of steroids was ad-ministered more than 7 days previously because of theshort-term benefits to the fetal lungs. They do, however,warn about the possibility of decreased birthweight andhead circumference at birth, which has been reported.For example, repeat antenatal steroid courses in fetalsheep result in increased lung maturation as well asincreased growth restriction. (13) Guinn and colleagues
(16) showed that the composite neonatal morbidity,including severe RDS, bronchopulmonary dysplasia(BPD), severe IVH, PVL, sepsis, necrotizing enterocoli-tis, or perinatal death, was not reduced by using weeklycourses as compared with one course of antenatal ste-roids. Because the true risk-to-benefit ratio of usingrepeat doses of antenatal steroids is not known, the1994 and 2000 NIH Consensus Conference recom-mends the use of repetitive courses of steroids only in thecontext of randomized, controlled trials (Table 5). (13)
SurfactantSRT was approved for use by the United States Food andDrug Administration in 1990. (5) Immediate improve-ment in oxygenation, along with improved aeration onchest radiograph within 1 hour, is seen after administra-tion of SRT. (5)(17) SRT reduces the incidence of RDS,death, pneumothorax, pulmonary interstitial emphy-sema (PIE), and IVH in preterm infants. (17) Althoughmost available evidence suggests that SRT increases sur-vival rates without increasing the risk of disability, the riskof long-term disability is unknown due to few reported
Figure. Classic chest radiograph in an infant who has RDS.
Table 5. Summary of 1994 and2000 National Institutes ofHealth Consensus ConferenceAntenatal SteroidRecommendations1. The benefits of prenatal corticosteroids outweigh
any risks that have been identified. The benefitsinclude decreased death and decreased incidence ofrespiratory distress syndrome and intraventricularhemorrhage.
2. All fetuses at 24 to 34 weeks’ gestation arecandidates for corticosteroid therapy.
3. Prenatal corticosteroid therapy should be usedwithout consideration of fetal sex, race, or theavailability of surfactant treatments for respiratorydistress syndrome.
4. Prenatal corticosteroids should be administered iftocolytics are used.
5. Because of probable benefit for treatment todelivery intervals of less than 24 hours, prenatalcorticosteroids are indicated unless delivery isimminent.
6. Repeated courses of corticosteroids may not be safeand should not be administered outside of clinicaltrials.
Reprinted with permission from Jobe. (13)
core concepts respiratory distress syndrome
NeoReviews Vol.10 No.7 July 2009 e355
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
follow-up studies on the preterm infants who have re-ceived surfactant. (17)
Surfactant is administered directly into the lungs viaan endotracheal tube. (5) Other methods of surfactantadministration, including aerosolization, nebulization,and instillation via bronchoalveolar lavage, have beenfound to be ineffective. (5) Surfactant administration vialaryngeal mask airway is being studied. (5) Surfactant canbe administered as either two or four fractional doses ineither two or four different body positions; clinical evi-dence is not sufficient to recommend an optimal numberof fractional doses. (17) Surfactant can be administeredas either a bolus or an infusion into the endotrachealtube; again, data in humans are insufficient to recom-mend an optimal method of surfactant administration.(17) Interestingly, data examining the distribution ofsurfactant in mechanically ventilated rabbits showed thatbolus instillation resulted in reasonably homogenous pul-monary surfactant distribution, while tracheal infusion re-sulted in extremely uneven pulmonary distribution. (18)
Natural and synthetic surfactant preparations exist,and both are effective in the treatment and prevention ofRDS. (19) Natural surfactants are derived from animallungs (bovine or porcine) and contain phospholipidswith SP-B and SP-C; first-generation synthetic surfac-tants contain only phospholipids without proteins. (19)A Cochrane meta-analysis by Soll and Blanco conductedin 2001 comparing natural surfactant to first-generationsynthetic surfactant confirmed that natural surfactantmore effectively reduces the risk of pneumothorax andlowers mortality rates in infants treated for RDS. (20)There is also a marginal decrease in the risk of BPD whenusing natural surfactant. Although natural surfactantsappear to be associated with higher rates of IVH, grade3 and 4 IVH rates are not increased. The conclusion ofthis meta-analysis is that natural surfactants are the moredesirable choice over the first-generation synthetic sur-factants, which is likely due to the inclusion of the SPs inthe natural surfactant. (20)
Synthetic surfactants containing peptides that mimicSPs recently have been developed and tested. (21) In ameta-analysis of two studies comparing protein-containing synthetic surfactant to natural surfactant, nostatistically significant differences were found betweenthe two groups in terms of death or chronic lung disease(CLD), and clinical outcomes were generally similar.(21) Further studies comparing these two groups areneeded.
The use of prophylactic versus selective administra-tion of surfactant has been studied thoroughly. Prophy-lactic SRT involves intubation and surfactant administra-
tion in preterm infants at high risk for RDS and usuallyoccurs after the initial resuscitation and within 10 to30 minutes of birth. (17) Prophylactic SRT has theadvantage of establishing a normal surfactant pool beforedamage due to a low FRC, and an increased work ofbreathing can occur. (5) Its major disadvantage is thepossibility that an infant who would not have developedRDS may be intubated and treated with surfactant. (5)Selective, or rescue, SRT is the administration of sur-factant to preterm infants who already have developedRDS. (17) The two types of selective SRT are early andlate. (17) Early selective SRT is administered within 1 to2 hours of birth; late selective SRT occurs 2 or morehours after birth. The advantage of selective SRT is theavoidance of overtreatment, but in those infants whodevelop RDS, the delay in treatment allows lung inflam-mation and damage to occur. (5)
In the Cochrane review by Soll and Morley in 2001,the use of prophylactic surfactant in infants at high risk ofdeveloping RDS was compared with selective surfactanttreatment at the time of respiratory failure. (22) Prophy-lactic surfactant treatment was associated with a signifi-cant reduction in the risk of pneumothorax, PIE, mor-tality, and BPD or death. (22) A secondary analysis ofinfants of fewer than 30 weeks’ gestation found a signif-icant decrease in the risk of mortality and the risk of BPDor death. The conclusion of this study is that prophylacticsurfactant is beneficial in preterm infants believed to be athigh risk for developing RDS, but the best method ofdetermining if an infant is at high risk for developingRDS remains unclear. (22)
Because the incidence of RDS decreases with increas-ing gestational age, it becomes likely that prophylactictreatment with surfactant once gestational ages approach28 to 30 weeks results in a good percentage of overtreat-ment. (5) In these cases, it may make more sense to treatselectively with surfactant. The most recent Cochranereview examining early versus late selective surfactantadministration found that early selective SRT decreasedneonatal mortality, pneumothoraces, PIE, and the inci-dence of CLD and death at 36 weeks’ postmenstrual agewhen compared with late selective SRT. (5)
Finally, in 1999, a Cochrane review compared multi-ple versus single doses of natural surfactant for the treat-ment of RDS. (23) The reason for this comparison wasthe observation that some infants seemed to relapseafter initial surfactant treatment. In this meta-analysis, amore sustained response in the treatment of RDS wasseen in the group of infants allowed to have multipledoses of surfactant. (23) A decreased risk of pneumotho-
core concepts respiratory distress syndrome
e356 NeoReviews Vol.10 No.7 July 2009
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
rax and a trend toward a decreased risk of mortality alsowas reported.
Overall, survival without BPD has increased sinceSRT began, although the incidence of BPD in verylow-birthweight infants is unchanged. (17) The risk ofrespiratory problems later in infancy or childhood (in-cluding asthma and infection) remains high for preterminfants who were treated with surfactant and mechanicalventilation. (17) Long-term studies are needed to assessthe respiratory function of children who received surfac-tant as preterm infants. (17)
Antenatal Steroids and SurfactantNo randomized, controlled trials have been conductedto address whether antenatal steroids reduce the need forprophylactic or rescue SRT in preterm infants. (17) Onsubgroup analyses of observational studies and clinicaltrials, infants born before 32 weeks’ gestation who re-ceived both antenatal steroids and SRT had significantreductions in mortality, severity of respiratory distress,and frequency of air leaks compared with infants whoreceived neither treatment, only antenatal steroids, oronly SRT. (17) Infants born before 27 weeks’ gestationdid not have a lower incidence of RDS, but the severity ofRDS may have been decreased. Therefore, it is generallyaccepted that the effects of antenatal steroids and SRTare additive, and it is not expected that trials will beconducted to verify this.
Ventilatory ManagementSeveral methods can be used to ventilate the pretermneonate at risk for RDS. Surfactant administration fol-lowed by conventional ventilation has historically beenthe management of choice, but concerns that both pos-itive pressure ventilation via the endotracheal tube andthe duration of mechanical ventilation have direct effectson the incidence of BPD have prompted investigators tosearch for less harsh ventilatory strategies. (24)(25) Be-cause most preterm infants who have RDS require ven-tilatory support and BPD is a major morbidity of manyforms of ventilatory support, the hope is to find a nonin-vasive method of ventilation for RDS that is both safe andeffective.
The initial belief was that more complex ventilationstrategies, such as high-frequency oscillatory ventilation,might decrease the risk of developing BPD. However,when optimal lung volume strategies are used, there is nodifference between conventional ventilators and high-frequency ventilators in terms of pulmonary and nonpul-monary outcomes. (24)(25) A Cochrane review on this
subject from 2007 confirmed the lack of clear evidencefor elective use of high-frequency ventilation over con-ventional ventilation because no difference was docu-mented in mortality between the two modes of ventila-tion at 30 days or at term-equivalent age. (26) Patient-triggered ventilation is a form of conventional ventilationthat includes synchronized intermittent mandatory ven-tilation, assist control, and pressure support. (24)(25)Studies have shown that patient-triggered ventilation hasbenefits over conventional ventilation and high-frequency ventilation in terms of a decreased duration ofmechanical ventilation and decreased number of days onoxygen. (24)(25) However, there was no significantdifference in terms of a decrease in lung injury betweenthe three ventilation strategies.
The noninvasive ventilation strategy of nCPAP is be-lieved to work by improving oxygenation without in-creasing PaCO2 through the stabilization and recruitmentof collapsed alveoli. (27) The idea is that nCPAP will helpto achieve the adequate FRC that is necessary to avoidthe development of RDS because increased FRC meansincreased alveolar surface area and less intrapulmonaryshunt. (27) The avoidance of endotracheal intubationsaves the infant from the barotrauma and volutraumaseen with the use of mechanical ventilators. A CochraneReview from 2002 states that although a higher rate ofpneumothorax was seen, there was an overall reductionin respiratory failure and mortality in preterm infantswho had RDS and were treated with nCPAP. (28) Largerandomized, controlled trials to evaluate this possibilityare underway.
The COIN trial (Continuous Positive Airway Pres-sure or Intubation at Birth) is a recently published ran-domized trial addressing whether the use of nCPAPshortly after birth would decrease the rates of death andBPD (defined as the need for oxygen at 36 weeks gesta-tional age). (29) A total of 610 infants from gestationalages 25 to 28 and 6/7 weeks were randomized at 5 min-utes after birth to receive either nCPAP or intubationand mechanical ventilation. Outcomes between the twogroups were assessed at 28 days, 36 weeks gestationalage, and before discharge. There was a significantly lowerrisk of death or need for oxygen at 28 days in thenCPAP-treated infants, but early nCPAP did not signif-icantly decrease the rates of death or BPD compared withintubation and ventilation at 36 weeks gestational age.Infants in the nCPAP group required fewer overall daysof ventilation, but also had a significant increase in pneu-mothoraces compared with mechanically ventilated in-fants. The overall conclusion of the study was that early
core concepts respiratory distress syndrome
NeoReviews Vol.10 No.7 July 2009 e357
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
nCPAP was not detrimental to preterm infants whosegestational ages were between 25 and 28 and 6/7 weeks.(29)
The SUPPORT trial (Surfactant Positive Airway Pres-sure and Pulse Oximetry Trial in Extremely Low Birth-weight Infants) is currently ongoing. This trial is ran-domizing infants of gestational ages between 24 weeksand 27 and 6/7 weeks to either a treatment group ofCPAP and permissive ventilation management or a con-trol group of prophylactic/early surfactant and conven-tional ventilator management as well as either a low (85%to 89%) or high (91% to 95%) SpO2 group. Results of thisstudy are pending. (30)
Finally, nasal intermittent mandatory ventilation(NIMV) has been studied in the treatment of RDS. Therationale for NIMV use is that the administration of“sighs” to the neonate can help to open microatelectasisand recruit more alveoli. (31) Kugelman and associates(31) showed that NIMV was more successful thannCPAP in the initial treatment of RDS among infantsyounger than 35 weeks’ gestation by reducing the rate ofendotracheal intubation and the incidence of BPD. (31)As with other studies, failure of nasal respiratory sup-port was associated with lower birthweight. Furtherstudy is needed, but NIMV may be a promising non-invasive method of ventilation for preterm infants at riskfor RDS.
Ventilatory Management and SurfactantSurfactant is a proven treatment for RDS that must beadministered via endotracheal tube. As ventilation strat-egies become more sophisticated and less invasive, theuse of surfactant may become more complicated. Thepush toward less invasive ventilation strategies does notallow an opportunity for surfactant administration. Toallow for use of surfactant, many centers have started tointubate, administer surfactant, and extubate to mini-mally invasive respiratory support (“in-and-out surf”).A recent Cochrane Review compared early surfactantadministration with brief ventilation to selective surfac-tant with continued mechanical ventilation in preterminfants who had or were at risk for RDS. (32) The use ofsurfactant followed by early extubation to nCPAP wasmore effective than selective intubation and surfactantadministration followed by mechanical ventilation in pre-venting the need for mechanical ventilation as well asdecreasing the incidence of BPD and pneumothorax.(32) No investigators currently are examining the use ofearly and continuous nCPAP versus surfactant adminis-tration followed by early extubation and nCPAP.
Inhaled Nitric Oxide (iNO)NO is a vascular endothelial relaxing factor that success-fully causes local smooth muscle cell relaxation in thepulmonary circulation when delivered by inhalation.(33) iNO has been used as a treatment in illnesses inwhich pulmonary vasodilation would be of benefit. Pul-monary hypertension is recognized as a complicatingfactor that may contribute to RDS. (33) The ability ofiNO to dilate the blood vessels in the pulmonary vascu-lature, reduce pulmonary hypertension, and improveventilation-perfusion matching (a known problem inRDS) has led to clinical trials on the use of iNO in thepreterm infant who has RDS. (33) Of note, one majorconcern for the use of iNO in preterm infants is theadverse effect of bleeding complications.
In the Cochrane Review conducted by Barringtonand Finer in 2007, iNO possibly improved outcome inmildly ill infants, with a possible decrease in ICH. (34)However, when administered to very ill preterm infants,iNO did not improve outcome and may have contrib-uted to an increase in ICH. (34) Overall, the benefit ofiNO in the preterm infant is largely unknown, and fur-ther randomized, controlled trials with subsequent meta-analyses are needed to answer this question. (33)
OtherSeveral other therapies have been studied as possibletreatments for RDS. The following therapies have beenreviewed in meta-analyses and published in the CochraneDatabase of Systematic Reviews.
DIURETICS. RDS may be complicated by lung edema,so studies have been performed to determine if adminis-tration of diuretics may improve the course of RDS.A Cochrane Review by Brion and Soll in 2007 (35)analyzed seven studies with the aim of assessing risks andbenefits of diuretic use in preterm infants who had RDS.Six of these studies used furosemide and were conductedbefore the era of prenatal steroids and surfactant. Al-though a transient furosemide-induced improvement inpulmonary function was seen, this benefit did not out-weigh the risk for patent ductus arteriosus and hemody-namic instability. There were no long-term benefits. Theother study assessed theophylline use and found no long-term benefits. Overall, the reviewers of these studies didnot find data to support the routine administration offurosemide or theophylline in preterm infants who hadRDS. (35)
ANTITHROMBIN (AT). AT is produced by the liver andis important in both blood clotting and clot lysis. Infants
core concepts respiratory distress syndrome
e358 NeoReviews Vol.10 No.7 July 2009
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
who have RDS, as well as infants who have other criticalillnesses, have low serum AT concentrations. It was hy-pothesized that increased thrombin formation due tolow AT concentrations might contribute to the patho-physiology of RDS and that administration of AT mayimprove the clinical course of affected infants. A reviewby Bassler and associates (36) found a trend towardincreased mortality as well as a significantly prolongedduration of mechanical ventilation and oxygen therapy inthe AT-treated group. Therefore, due to the lack ofbenefit, as well as the potential harm, AT is not a recom-mended treatment for infants who have RDS.
DIGOXIN. It has been suggested that pulmonaryedema due to congestive heart failure may contribute toRDS in the neonate. Based on this suggestion, digoxinhas been studied as a potential treatment in RDS. Tworandomized, controlled trials were analyzed by Soll, (37)who found that digoxin did not result in improved RDSsymptoms. Therefore, digoxin is not recommended foruse in infants solely affected with RDS.
INOSITOL. Inositol is a nutrient required by cells forgrowth and survival that also has been found to promotematuration of several components of surfactant. A 2003review by Howlett and Ohlsson (38) includes threerandomized, controlled trials of the use of inositol inpreterm infants who had RDS. A significant reduction indeath or BPD, stage 4 retinopathy of prematurity, andgrade 3 or 4 IVH was seen in the inositol-treated group.No significant increase in adverse effects was reported.Due to the relatively small number of infants in thesereviewed trials, multicenter randomized, controlled trialsare recommended. However, these early results onthe use of inositol in preterm infants with RDS arepromising.
POSTNATAL THYROID HORMONE. Animal research hasshown that antenatal administration of thyroid hormonestimulates surfactant production and reduces the inci-dence and severity of RDS. A review by Osborn andHunt (39) examined trials that used postnatal thyroidhormone in preterm infants who had RDS. The conclu-sion was that administration of thyroid hormone therapywithin the first hours after birth had no significant effecton the severity of RDS, morbidity, or mortality in suchpreterm infants and, therefore, is not recommended.
Complications and Treatment of RDSA major pulmonary complication of RDS is the develop-ment of BPD, which is generally defined as the need for
oxygen supplementation at 36 weeks’ corrected gesta-tional age. (11) Importantly, BPD is not caused by RDS;rather, it can be the result of the many treatments ofRDS. (40) The “new BPD,” a term coined by Jobe in1999, describes a syndrome that results from processesthat interfere with lung development, not a syndromeresulting only from injury. (40) These processes caninclude chorioamnionitis, oxygen administration, hightidal volumes, mechanical ventilation, postnatal sepsis,and postnatal corticosteroids. Accordingly, it is possibleto develop BPD without having RDS, but BPD abso-lutely can occur in preterm infants who developed andwere treated for RDS. (40) Other complications of RDSin the preterm infant include IVH, patent ductus arteri-osus, sepsis, and pulmonary hemorrhage, which likelyresult from a combination of prematurity, RDS, and itstreatments.
Complications from the treatments for RDS are inev-itable, but based on risk-to-benefit ratios of the treat-ments, the complications are mostly tolerable. Antenatalsteroids do not have true short-term complications whenexamined in meta-analyses; there has been no associatedincrease in maternal death, maternal infection, fetaldeath, neonatal CLD, or neonatal birthweight. (1) Con-cerns of decreased birthweight (15) as well as trendstoward increased incidence of IVH and long-term ad-verse behaviors have been voiced with the use of multiplerepeat doses of antenatal steroids, but never consistentlyproven. (16) Interestingly, in a 30-year follow-up ofinfants who received antenatal corticosteroids, no changein adult size or blood lipid or cortisol concentrations wasdoumented, but there was a slight increase in the inci-dence of insulin resistance. (13) These results may haveimplications for the hypothesis of the fetal origins ofadult disease. (1)
Mild complications of surfactant administration mayinclude transient oxygen desaturation, apnea, and brady-cardia, but such complications typically improve rapidly.(5) More serious complications include endotrachealtube blockage and pulmonary hemorrhage. (5) Afteradministration, surfactant may distribute unevenly toonly one lung or certain lobes. A second dose generallyfollows the same course as the first, which can lead tocontinued atelectasis of certain areas of the lungs. (9) Asmentioned, natural surfactant administration causes anincrease in grade 1 and 2 IVH compared with syntheticsurfactant. (20) Finally, after surfactant administration,the clinical signs of a PDA may develop earlier in theclinical course. (17)
Complications of mechanical ventilation are not spe-cific to infants being treated for RDS. Air leak syn-
core concepts respiratory distress syndrome
NeoReviews Vol.10 No.7 July 2009 e359
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
dromes, including PIE and pneumothorax, are morecommon when the poorly compliant lungs in RDS aremechanically ventilated. (2) Pneumothorax is also asso-ciated with the use of nCPAP. (29)
Long-term PrognosisSurvival of infants who have RDS has improved greatlywith the use of antenatal steroids and SRT. Preliminarydata in infants treated with antenatal steroids suggestthe possibility of less neurodevelopmental delay. (1)Overall, however, information regarding neurodevelop-mental outcomes in the preterm infants treated for RDSis lacking, and long-term follow-up studies are needed.
References1. Roberts D, Dalziel S. Antenatal corticosteroids for acceleratingfetal lung maturation for women at risk of preterm birth. CochraneDatabase Syst Rev. 2006;3: CD0044542. Kopelman AE, Matthew OP. Common respiratory disorders ofthe newborn. Pediatr Rev. 1995;16:209–2173. American Lung Association. Respiratory Distress Syndrome of theNewborn Fact Sheet. 2008. Available at: http://www.lungusa.org/site/apps/nlnet/content3.aspx?c�dvLUK9O0E&b� 2060721&content_id�{552A7003-4621-43E5-82B4-1678D9A6D963}¬oc�1. Accessed August 20084. Fraser J, Walls M, McGuire W. ABC of preterm birth: respira-tory complications of preterm birth. Br Med J. 2004;329:962–9655. Stevens TP, Sinkin RA. Surfactant replacement therapy. Chest.2007;131:1577–15826. Hallman M. Lung surfactant, respiratory failure, and genes.N Engl J Med. 2004;350:1278–12807. Aly H. Respiratory disorders in the newborn: identification anddiagnosis. Pediatr Rev. 2004;25:201–2088. Schreiner RL, Bradburn NC. Newborns with acute respiratorydistress: diagnosis and management. Pediatr Rev. 1988;9:279–2859. Jobe AH. Why surfactant works for respiratory distress syn-drome. NeoReviews. 2006;7:e95–e10610. Joshi S, Kotecha S. Lung growth and development. EarlyHuman Dev. 2007;83:789–79411. Rodriguez RJ. Management of respiratory distress syndrome:an update. Respir Care. 2003;48:279–28712. Tumbaga PF, Philip AGS. Perinatal group B streptococcalinfections: past, present, and future. NeoReviews. 2003;4:e65–e72
13. Jobe AH. Prenatal corticosteroids: a neonatologist’s perspec-tive. NeoReviews. 2006;7:e259–e26814. Baud O, Foix-L’Helias L, Kaminski M, et al. Antenatal glu-cocorticoid treatment and cystic periventricular leukomalacia invery premature infants. N Engl J Med. 1999;341:1190–119615. Crowther CA, Harding JE. Repeat doses of prenatal cortico-steroids for women at risk of preterm birth for preventing neonatalrespiratory disease. Cochrane Database Syst Rev. 2007;3: CD00393516. Guinn DA, Atkinson MW, Sullivan L, et al. Single vs weeklycourses of antenatal corticosteroids for women at risk of pretermdelivery. JAMA. 2001;286:1581–158717. Engle WA, Committee on Fetus and Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and termneonate. Pediatrics. 2008;121:419–43218. Segerer H, van Gelder W, Angenent FWM, et al. Pulmonarydistribution and efficacy of exogenous surfactant in lung-lavagedrabbits are influenced by the instillation technique. Pediatr Res.1993;34:490–49419. Moya F, Maturana A. Animal-derived surfactants versus pastand current synthetic surfactants: current status. Clin Perinatol.2007;34:145–17720. Soll RF, Blanco F. Natural surfactant extract versus syntheticsurfactant for neonatal respiratory distress syndrome. CochraneDatabase Syst Rev. 2001;2:CD00014421. Pfister RH, Soll RF, Wiswell T. Protein containing syntheticsurfactant versus animal derived surfactant extract for the preven-tion and treatment of respiratory distress syndrome. CochraneDatabase Syst Rev. 2007;4:CD00606922. Soll RF, Morley CJ. Prophylactic versus selective use of surfac-tant in preventing morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2001;2:CD00051023. Soll RF. Multiple versus single dose natural surfactant extractfor severe neonatal respiratory distress syndrome. Cochrane Data-base Syst Rev. 1999;2:CD00014124. Ramanathan R, Sardesai S. Lung protective ventilatory strate-gies in very low birth weight infants. J Perinatol. 2008;28:S41–S4625. Ramanathan R. Optimal ventilatory strategies and surfactant toprotect the preterm lung. Neonataolgy. 2008;93:302–30826. Henderson-Smart DJ, Cools F, Bhuta T, Offringa M. Electivehigh frequency oscillatory ventilation versus conventional ventila-tion for acute pulmonary dysfunction in preterm infants. CochraneDatabase Syst Rev. 2007;3:CD00010427. Thomson MA. Early nasal continuous positive airway pressureto minimize the need for endotracheal intubation and ventilation.NeoReviews. 2005;6:e184–e18828. Ho JJ, Subramaniam P, Henderson-Smart DJ, Davis PG.Continuous distending pressure for respiratory distress syndrome inpreterm infants. Cochrane Database Syst Rev. 2002;2:CD00227129. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM,Carlin JB, for the COIN Trial Investigators. Nasal CPAP of intu-bation at birth for very preterm infants. N Engl J Med. 2008;358:700–70830. Surfactant Positive Airway Pressure and Pulse Oximetry Trial(SUPPORT). ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00233324. Accessed March 200931. Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B,Bader D. Nasal intermittent mandatory ventilation versus nasalcontinuous positive airway pressure for respiratory distress syn-drome: a randomized, controlled, prospective study. J Pediatr.2007;150:521–52632. Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant
American Board of Pediatrics Neonatal-PerinatalMedicine Content Specifications● Know the pathophysiology and risk factors
for RDS.● Recognize the clinical, imaging, and
laboratory features of RDS.● Recognize the pathologic features of RDS.● Know the clinical strategies and therapies used to decrease
the risk and severity of RDS.● Know the management of RDS, including surfactant
replacement.
core concepts respiratory distress syndrome
e360 NeoReviews Vol.10 No.7 July 2009
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
administration with brief ventilation vs. selective surfactant andcontinued mechanical ventilation for preterm infants with or at riskfor respiratory distress syndrome. Cochrane Database Syst Rev.2007;4:CD00306333. Van Meurs KP. Inhaled nitric oxide therapy in the preterminfant who has respiratory distress syndrome. NeoReviews. 2005;6:e268–e27734. Barrington KJ, Finer NN. Inhaled nitric oxide for respiratoryfailure in preterm infants. Cochrane Database Syst Rev. 2007;3:CD00050935. Brion LP, Soll RF. Diuretics for respiratory distress syndromein preterm infants. Cochrane Database Syst Rev. 2008;1:CD001454
36. Bassler D, Millar D, Schmidt B. Antithrombin for respiratorydistress syndrome in preterm infants. Cochrane Database Syst Rev.2006;4:CD00538337. Soll RF. Digoxin for preventing or treating neonatal respira-tory distress syndrome. Cochrane Database Syst Rev. 1998;2:CD00108038. Howlett A, Ohlsson A. Inositol for respiratory distress syn-drome in preterm infants. Cochrane Database Syst Rev. 2003;4:CD00036639. Osborn DA, Hunt RW. Postnatal thyroid hormones for respi-ratory distress syndrome in preterm infants. Cochrane Database SystRev. 2007;1:CD00594640. Jobe AH. The new BPD. NeoReviews. 2006;7:e531–e545
NeoReviews Quiz
10. Normal lung development during fetal life occurs through a series of sequential phases that leads to amature lung with a large surface area and a minimal diffusion distance for gas exchange. Of thefollowing, the presence of respiratory bronchioles, alveolar ducts, and primitive alveoli in the developinglung is most characteristic of the:
A. Alveolar phase.B. Canalicular phase.C. Embryonic phase.D. Pseudoglandular phase.E. Saccular phase.
11. In addition to phospholipids, pulmonary surfactant contains four major proteins: surfactant protein (SP)-A, SP-B, SP-C, and SP-D. Each surfactant protein has a specific function. Of the following, SP-B is mostimportant for:
A. Facilitating formation of tubular myelin.B. Participating in host innate immune defense.C. Promoting adsorption and spreading of surfactant.D. Regulating surfactant reuptake and recycling.E. Regulating surfactant secretion and uptake.
12. Surfactant replacement therapy has been approved by the United States Food and Drug Administration forthe treatment of respiratory distress syndrome (RDS) since 1990. Several other therapeutic approacheshave been studied as possible adjunct treatments for RDS, as reviewed in meta-analyses published in theCochrane Database of Systematic Reviews. Of the following, the most promising adjunct treatment forRDS in preterm infants is the administration of:
A. Antithrombin.B. Digoxin.C. Furosemide.D. Inositol.E. Thyroxin.
core concepts respiratory distress syndrome
NeoReviews Vol.10 No.7 July 2009 e361
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
DOI: 10.1542/neo.10-7-e3512009;10;e351Neoreviews
Jamie B. Warren and JoDee M. AndersonCore Concepts : Respiratory Distress Syndrome
ServicesUpdated Information &
http://neoreviews.aappublications.org/content/10/7/e351including high resolution figures, can be found at:
References
http://neoreviews.aappublications.org/content/10/7/e351#BIBLat: This article cites 23 articles, 12 of which you can access for free
Subspecialty Collections
disordershttp://neoreviews.aappublications.org/cgi/collection/respiratory_Respiratory Disordersorn_infanthttp://neoreviews.aappublications.org/cgi/collection/fetus_newbFetus and Newborn Infantfollowing collection(s): This article, along with others on similar topics, appears in the
Permissions & Licensing
/site/misc/Permissions.xhtmltables) or in its entirety can be found online at: Information about reproducing this article in parts (figures,
Reprints/site/misc/reprints.xhtmlInformation about ordering reprints can be found online:
at Health Sciences Library State Univ Of New York on August 13, 2012http://neoreviews.aappublications.org/Downloaded from
Related Documents