CHANGES IN THE ENDOCANNABINOID SYSTEM IN THE BRAIN FOLLOWING BILATERAL VESTIBULAR DAMAGE Jean-Ha Baek A thesis submitted for the degree of Doctor of Philosophy at the University of Otago, Dunedin New Zealand. December 2010

Welcome message from author
This document is posted to help you gain knowledge. Please leave a comment to let me know what you think about it! Share it to your friends and learn new things together.
Transcript

CHANGES IN THE ENDOCANNABINOID SYSTEM
IN THE BRAIN FOLLOWING BILATERAL VESTIBULAR DAMAGE
Jean-Ha Baek
A thesis submitted for the degree of
Doctor of Philosophy
at the University of Otago, Dunedin
New Zealand.
December 2010

ii

Dedication iii
To my parents,
who are my mentors and my inspiration
Fear of the LORD is the beginning of wisdom.
Knowledge of the Holy One results in understanding.
<Proverbs 9:10>

Abstract iv
ABSTRACT
Numerous studies have shown that bilateral vestibular deafferentation (BVD) results in
spatial memory deficits and hippocampal dysfunction in rats and humans. These deficits
appear to be long-lasting, suggesting long-term adverse effects on the hippocampus, a brain
region known for its role in learning and memory. Interestingly, the endocannabinoid system
has also been demonstrated to have a role in modulating hippocampal synaptic
neurotransmission and cognitive function. Not only are the cannabinoid receptors highly
expressed in the hippocampus, the activation of these receptors by either the endogenous
ligands (that is, endocannabinoids) or exogenous cannabinoid drugs results in the impairment
in a variety of learning and memory tasks. As it has been demonstrated that vestibular damage
causes learning and memory deficits and that the endocannabinoid system is critically
involved in the mechanism of learning and memory, the aim of this study was to investigate
whether the hippocampal endocannabinoid system plays a role in the functional changes seen
after vestibular damage.
By manipulating the available cues in the foraging task, 14 months post-BVD animals
were unable to use available visual cues and execute piloting as their navigating strategy. In
addition, their spatial navigation ability became worse in darkness. Importantly, it appears that
these cognitive impairments due to the vestibular damage are highly likely to be permanent. It
was expected that the administration of the cannabinoid receptor agonist would exacerbate the
observed spatial memory deficits in the BVD animals as it has been shown to have disruptive
effects on spatial learning and memory in both animals and humans. In the present study, it
was found that 1 mg/kg WIN55212-2 (WIN), a cannabinoid receptor agonist, was sufficient to
produce object recognition memory impairments in adult rats. However, when the same drug
was given to both the BVD and the sham animals, its effect on spatial memory was complex.
It was shown that WIN, at 2 mg/kg, significantly improved the performance in the dark in
BVD, but not sham animals, suggesting that BVD might have resulted in changes in the
brain‟s endocannabinoid system. However, the pre-treatment with AM251, a cannabinoid
receptor inverse agonist, further indicated the complexity of involvement of the
endocannabinoid system in the cognitive deficits following BVD.
Since cannabinoid cannabinoid 1 (CB1) receptors are well known to regulate synaptic
plasticity in the hippocampus, whether BVD resulted in changes in CB1 receptor expression

Abstract v
and affinity in the rat hippocampus at 1, 3 and 7 days post-surgery was investigated, using a
combination of western blotting and radioligand binding. It was found that the CB1 receptor
down-regulates in the CA3 region of the hippocampus following BVD, but with no changes
in the affinity of the CB1 receptor for WIN.
Overall, the results from the present study suggest that cognitive deficits following
BVD may be permanent and provide some preliminary evidence for future research on the
role of the endocannabinoid system in the observed cognitive deficits following vestibular
damage in order to further elucidate the potential mechanism(s) that may underlie this
impairment.

Acknowledgements vi
ACKNOWLEDGEMENTS
Throughout the course of this PhD study, I have been very fortunate to have so many
people who have guided me, and have enabled me to reach where I am now. I am forever
grateful for their encouragement and support.
Most of all, I would like to give my sincere gratitude to each of my supervisors, Prof.
Paul Smith, Dr. Yiwen Zheng, and Assoc. Prof. Cynthia Darlington. Aristotle once said,
“Those who wish to succeed must ask the right preliminary questions.” Each of my
supervisors has taught me exactly that – how to ask the right questions at in order to reveal
what is unknown.
I thank Paul deeply for being a fantastic supervisor and a good mentor. Paul was always
been there when I needed guidance. Paul has shown me how to approach research problems in
different ways and how to be both critical and philosophical about my work. Paul‟s
enthusiasm towards research will always serve as a reminder to me of how to enjoy science. I
also thank Paul for his ability to pick out the difference between when to use a comma and
when to use a semi-colon. His help in editing my writing has been tremendous. Huge thanks
are also due to Yiwen, whose contribution has been more than I can write on this page. She
has taught me almost all of my experimental skills, and she is an amazing teacher. Her depth
of knowledge is beyond measure. Yiwen always had answers to my “inexperienced” questions,
whether they were science-related or not. Thank you also to Cynthia, who has been a warm
influence during my PhD. She always had confidence in me when I doubted myself, and
brought out the good things in me. Cynthia has also taught me how to indulge myself in
research while keeping everything “cool”.
I am extremely thankful to Dr. Lisa Geddes who has helped me enormously to tie the
individual pieces together to make a “story” out of this thesis. I cannot imagine what it would
have been like without her objective and critical inputs towards this work. I am thankful that I
had a chance to get to know her in the last few months of PhD. Many thanks also to my PhD
buddy, Sangeeta Balabhadrapatruni, for her Tender Loving Care and for being a good listener.
I am very grateful for our friendship. Thank you also to my wonderful colleagues in the
Smith/Darlington/Zheng research group (also known as The Centre of Excellence in
Vestibular Research); Shaeza Begum, Irene Cheung, Emma Hamilton, Emily McNamara,

Acknowledgements vii
Lucy Stiles, Shweta Vagal, Georgina Wilson, and Jun Yoon for making my life in the lab
joyful and exciting. Many thanks are due to everyone who has contributed towards this work
in their own special ways, including people in the Department of Pharmacology and
Toxicology, many friends from Korean Evergreen Church in Dunedin, and from Saeronam
Church in Korea.
Sincere thanks to Dad, who has given me priceless advice on all of my experiments and
on my attitude towards scientific research. Despite his unexpected illness, he continued to
encourage me to do my best. Dad has shown me how to persevere in tough times, to be
humble, and to work diligently. Not only has he been a perfect Dad but also an amazing
scientist. Although God took Dad away towards the very end of this study, I know from the
bottom of my heart that God still loves me and that His amazing grace will continue to be
with me as it has been throughout the course of this study. Many thanks to Mum for all her
prayers, and for always giving me hope and strength to carry on with this study. I also thank
my brother for our valuable and memorable times that we had together in Dunedin.

List of Publications viii
LIST OF PUBLICATIONS
1) Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2008) Cannabinoid CB2 receptor
expression in the rat brainstem cochlear and vestibular nuclei. Acta Otolaryngol, 128,
961-967.
2) Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2009) The CB1 receptor agonist,
WIN 55,212-2, dose-dependently disrupts object recognition memory in adult rats.
Neurosci Lett, 464, 71-73.
3) Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2010) Evidence that spatial memory
deficits following bilateral vestibular deafferentation in rats are probably permanent.
Neurobiol Learn Mem, 94, 402-413.
4) Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2010, in press) Cannabinoid CB1
receptor expression and affinity in the rat hippocampus following bilateral vestibular
deafferentation. Neurosci Lett.
5) Smith, P.F., Geddes, L.H., Baek, J.-H., Darlington, C.L., and Zheng, Y. (2010) Modulation
of memory in humans by vestibular lesions and galvanic vestibular stimulation. Front
Neurology, 1(141): 1-8 (doi: 10.3389/fneur.2010.00141).

Table of Contents ix
TABLE OF CONTENTS
Dedication ······································································································································ iii
ABSTRACT ······································································································································ iv
ACKNOWLEDGEMENTS·················································································································· vi
LIST OF PUBLICATIONS················································································································ viii
TABLE OF CONTENTS ····················································································································· ix
LIST OF FIGURES AND TABLES ···································································································· xiv
LIST OF ABBREVIATIONS ·············································································································· xx
CHAPTER 1: INTRODUCTION ········································································································· 1
1.1 THE VESTIBULAR SYSTEM ······························································································· 3
1.1.1 Anatomical Structure and Function ································································· 3
1.1.2 Vestibular Pathways ························································································ 7
1.1.3 Peripheral Vestibular Deafferentation ····························································· 9
1.1.4 Vestibular-Hippocampal Connections ··························································· 11
1.2 THE ENDOCANNABINOID SYSTEM ·················································································· 19
1.2.1 Cannabinoid Receptors and Endocannabinoids ············································ 19
1.2.2 Neuromodulatory Role of the Endocannabinoid System ································· 25
1.2.3 The Role of Endocannabinoid System in Cognitive Function ························· 27
1.3 THE ASSOCIATION BETWEEN THE VESTIBULAR AND ENDOCANNABINOID SYSTEMS ······ 30
1.4 RESEARCH GOALS ········································································································· 31
CHAPTER 2: THE EFFECT OF THE CANNABINOID RECEPTOR AGONIST, WIN 55212-2, ON
OBJECT RECOGNITION MEMORY IN RATS ················································································· 32
2.1 INTRODUCTION ·············································································································· 33
2.2 MATERIALS AND METHODS···························································································· 36
2.2.1 Animals ········································································································· 36
2.2.2 Drugs ············································································································ 36
2.2.3 Object Recognition Memory Test ··································································· 36
2.2.4 Statistical Analysis ························································································ 37

Table of Contents x
2.3 RESULTS························································································································· 39
2.3.1 Total Exploration Time ·················································································· 39
2.3.2 Exploration Time by Objects ········································································· 40
2.3.3 Discrimination Index ····················································································· 41
2.4 DISCUSSION···················································································································· 42
CHAPTER 3: THE EFFECT OF CANNABINOIDS ON SPATIAL MEMORY FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS ················································································· 44
3.1 INTRODUCTION ·············································································································· 45
3.2 MATERIALS AND METHODS···························································································· 49
3.2.1 Animals ········································································································· 49
3.2.2 Peripheral Vestibular Lesion Surgery ···························································· 49
3.2.3 Drugs ············································································································ 50
3.2.4 Foraging Task Apparatus ·············································································· 50
3.2.5 Foraging Task Procedure ·············································································· 53
3.2.6 Data Acquisition ··························································································· 54
3.2.7 Statistical Analysis ························································································ 56
3.3 RESULTS························································································································· 58
3.3.1 General Behavioural Observations ······························································· 58
3.3.2 Pre-Training ································································································· 58
3.3.3 Light Probe Trial ··························································································· 60
3.3.4 Dark Training ······························································································· 64
3.3.5 AM251 Treatment ·························································································· 78
3.4 DISCUSSION···················································································································· 82
CHAPTER 4: CHANGES IN CB1 RECEPTOR DENSITY AND AFFINITY FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS ················································································· 93
4.1 INTRODUCTION ·············································································································· 94
4.2 MATERIALS AND METHODS···························································································· 97
4.2.1 Western Blotting

Table of Contents xi
4.2.1.1 Animals ······················································································· 97
4.2.1.2 Tissue Collection ········································································· 97
4.2.1.3 Tissue Homogenisation ································································ 97
4.2.1.4 Bradford Assay ············································································ 97
4.2.1.5 Western Blot ················································································ 98
4.2.1.6 Data Acquisition ········································································ 100
4.2.1.7 Statistical Analysis ····································································· 101
4.2.2 Radioligand Binding Assay
4.2.2.1 Animals ····················································································· 101
4.2.2.2 Cannabinoid Ligands ································································ 101
4.2.2.3 Membrane Preparation ······························································ 102
4.2.2.4 [3H]-CP 55,940 Binding Assay ·················································· 103
4.2.2.5 Data Acquisition and Statistical Analysis ··································· 104
4.3 RESULTS······················································································································· 105
4.3.1 Western Blotting
4.3.1.1 Optimum Sample Amount ·························································· 105
4.3.1.2 CB1 Receptor Antibody Specificity ············································· 106
4.3.1.3 Changes in CB1 Receptor Protein Density ································· 106
4.3.2 Radioligand Binding Assay
4.3.2.1 Optimisation of the Protein Amount and Radioligand Concentration
································································································· 108
4.3.2.2 Changes in the CB1 Receptor Binding Affinity ··························· 109
4.4 DISCUSSION···················································································································110
CHAPTER 5: DISCUSSION AND CONCLUSIONS ···········································································116
APPENDICES ································································································································ 123
APPENDIX 1: THE SPECIFICITY OF CANNABINOID CB1 RECEPTOR LABELLING IN THE RAT
HIPPOCAMPUS ····························································································································· 124
1.1 INTRODUCTION ············································································································ 124
1.2 MATERIALS AND METHODS·························································································· 127
1.2.1 Removal of Brain ························································································ 127
1.2.2 Tissue Sectioning························································································· 127

Table of Contents xii
1.2.3 Immunohistochemistry ················································································ 128
1.2.4 Double Label Immunofluorescence ····························································· 129
1.2.5 Data Acquisition ························································································· 130
1.3 RESULTS······················································································································· 131
1.3.1 Optimisation of the CB1 Receptor Primary Antibody ··································· 131
1.3.2 Optimisation of the Secondary Antibody ······················································ 133
1.3.3 Double Labelling Immunofluorescence························································ 135
1.4 DISCUSSION·················································································································· 137
APPENDIX 2: CHANGES IN CB1 RECEPTOR EXPRESSION FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS ··············································································· 139
2.1 INTRODUCTION ············································································································ 139
2.2 MATERIALS AND METHODS·························································································· 142
2.2.1 Animals ······································································································· 142
2.2.2 Immunohistochemistry ················································································ 142
2.2.3 Data Acquisition ························································································· 142
2.2.4 Statistical Analysis ······················································································ 142
2.3 RESULTS······················································································································· 143
2.3.1 Spatial Distribution of the CB1 Receptors in the Hippocampus ···················· 143
2.4 DISCUSSION·················································································································· 146
APPENDIX 3: THE SPECIFICITY OF CANNABINOID CB2 RECEPTOR LABELLING IN THE RAT
HIPPOCAMPUS ····························································································································· 147
3.1 INTRODUCTION ············································································································ 147
3.2 MATERIALS AND METHODS·························································································· 147
3.2.1 Animals ······································································································· 147
3.2.2 Western Blotting ·························································································· 147
3.2.3 Immunohistochemistry ················································································ 147
3.2.4 Double Label Immunofluorescence ····························································· 152
3.2.5 Data Acquisition ························································································· 153

Table of Contents xiii
3.3 RESULTS······················································································································· 154
3.3.1 Western Blotting ·························································································· 154
3.3.2 Immunohistochemical Analysis of the CB2-Specific Antibody······················· 154
3.3.3 Double Labelling Immunofluorescence························································ 158
3.4 DISCUSSION·················································································································· 161
REFERENCES ······························································································································· 163
The New Beginning···················································································································· 206

List of Figures and Tables xiv
LIST OF FIGURES AND TABLES
CHAPTER 1: INTRODUCTION
Figure 1.1 Venn diagram showing the possible association between the vestibular and
endocannabinoid systems, together with their discrete involvement in cognitive function and
synaptic plasticity ·················································································································· 2
Figure 1.2 A schematic representation of the structure of the vestibular labyrinth with its
innervating vestibular and cochlear nerves ············································································· 4
Figure 1.3 A schematic representation of the structure and organisation of different types of
hair cells in the vestibular sensory epithelium ········································································ 6
Figure 1.4 Simplified diagram representing the organisation of the key areas and pathways
for the production of VORs and VSRs ··················································································· 9
Figure 1.5 Some potential vestibulo-hippocampal connections ············································ 14
Table 1.1 Neurochemical changes in the hippocampus and in the neocortex following
vestibular damage ················································································································ 16
Figure 1.6 Schematic diagram of the synthesis, signalling, and degradation of
endocannabinoids AEA and 2-AG at the synaptic cleft ························································· 24
CHAPTER 2: THE EFFECT OF THE CANNABINOID RECEPTOR AGONIST, WIN 55212-2, ON
OBJECT RECOGNITION MEMORY IN RATS
Figure 2.1 The object recognition memory testing conditions for each day ·························· 38
Figure 2.2 Total exploration time (in seconds) for session 1 (before drug administration) and
session 2 (after drug administration) for the vehicle treated group and groups receiving 1, 3 or
5 mg/kg WIN ······················································································································· 39
Figure 2.3 Mean exploration time (in seconds) for familiar and novel objects for the vehicle-
treated group and the groups receiving 1, 3 or 5 mg/kg WIN ················································ 40
Figure 2.4 The discrimination indices for the vehicle-treated group and the groups receiving 1,

List of Figures and Tables xv
3 or 5 mg/kg WIN ················································································································ 41
CHAPTER 3: THE EFFECT OF CANNABINOIDS ON SPATIAL MEMORY FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS
Figure 3.1 The foraging task apparatus ················································································ 52
Figure 3.2 The display window of the custom-coded path tracing software on a PC············· 55
Figure 3.3 Schematic diagram of measuring the initial heading angle ·································· 55
Figure 3.4 Number of pre-training sessions required for the sham and BVD animals to reach
the foraging task criterion ···································································································· 58
Figure 3.5 Searching and homing time of the sham and BVD animals in the last 6 days of the
pre-training ·························································································································· 59
Figure 3.6 First and second home choices of the light probe trial ········································· 60
Figure 3.7 Foraging task parameters displayed by sham-vehicle, sham-WIN, BVD-vehicle,
and BVD-WIN animals in the light probe trial ····································································· 61
Figure 3.8 The velocity comparison between searching and homing in the light probe trial · 62
Figure 3.9 The average number of visits to the old home, and the average number of errors
made before reaching the correct home by sham-vehicle, sham-WIN, BVD-vehicle, and
BVD-WIN animals in the light probe trial ············································································ 63
Figure 3.10 Linear graph of the searching distance (cm) for different treatment groups during
the dark training and the AUC of the searching distance for the sham and BVD animals to
show the effect of surgery, dose and the interactions····························································· 64
Figure 3.11 Linear graph of the searching time (sec) for different treatment groups during the
dark training and the AUC of the searching time showing the effect of surgery, treatment, and
dose ····································································································································· 65
Figure 3.12 Linear graph of the searching velocity (cm/sec) for different treatment groups
during the dark training and the AUC of the searching velocity showing the effect of surgery,
treatment, and dose ·············································································································· 66

List of Figures and Tables xvi
Figure 3.13 An example of the homeward path taken by a sham and a BVD rat in the dark
training ································································································································ 67
Figure 3.14 Linear graph of the homing distance (cm) for different treatment groups during
the dark training and the AUC of the homing distance showing the effect of surgery, treatment,
and dose ······························································································································· 68
Figure 3.15 Linear graph of the homing time (sec) for different treatment groups during the
dark training and the AUC of the homing time showing the effect of surgery, treatment, and
dose ····································································································································· 69
Figure 3.16 Linear graph of the homing velocity (cm/sec) for different treatment groups
during the dark training and the AUC of the homing velocity showing the effect of surgery,
treatment, and dose ·············································································································· 70
Figure 3.17 The velocity comparison between searching and homing during the dark training..
············································································································································ 71
Figure 3.18 Rose diagram showing the initial heading angles of the sham and BVD animals
for the dark training ············································································································· 72
Figure 3.19 Initial heading angles of animals in different treatment groups for 1 and 2 mg/kg
WIN treatment ····················································································································· 73
Figure 3.20 First and second home choices of the sham and BVD animals with 1 and 2 mg/kg
WIN treatment in the dark training ······················································································· 74
Figure 3.21 Linear graph of the number of errors made before reaching the correct home by
animals in different treatment groups and the AUC of the number of errors for the sham and
BVD animals to show the effect of surgery, drug treatment, dose, and their interactions ······· 75
Figure 3.22 The number of animals that completed the task during the dark training ··········· 76
Figure 3.23 Simple regression analysis performed to predict the number of errors made by
vehicle-treated sham and BVD animals or by all of the sham and the BVD animals from their
searching velocities ·············································································································· 77
Figure 3.24 Foraging task parameters displayed by sham-vehicle, sham-WIN, BVD-vehicle,
and BVD-WIN animals with AM251 treatment on day 21 of the dark training ····················· 79

List of Figures and Tables xvii
Figure 3.25 The velocity comparison between searching and homing with AM251 treatment
on day 21 of the dark training ······························································································· 78
Figure 3.26 First and second home choices of the BVD and sham animals on day 21 of the
dark training with the treatment of AM251 prior to vehicle or WIN administration ·············· 80
Figure 3.27 Initial heading angles of vehicle- or WIN-treated animals on day 21 of the dark
training with the treatment of AM251 prior to vehicle or WIN administration ······················ 81
Figure 3.28 The graph showing the average number of errors made before reaching the
correct home by the sham-vehicle, sham-WIN, BVD-vehicle, and BVD-WIN animals on day
21 of the dark training ·········································································································· 81
CHAPTER 4: CHANGES IN CB1 RECEPTOR DENSITY AND AFFINITY FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS
Figure 4.1 The CB1 receptor band intensity increased as the amount of sample protein
increased. This change can be visualised in the western blots for the CB1 receptor and its
corresponding actin ············································································································ 105
Figure 4.2 Western blot images showing the concerning lack of specificity of the CB1
receptor antibody for CB1 receptors ··················································································· 106
Figure 4.3 The levels of CB1 receptor protein in the CA1, CA3, DG of the hippocampus at 1,
3, and 7 days following BVD compared to the sham controls ············································· 107
Figure 4.4 Saturation binding curve with increasing amount of hippocampal membrane
preparation for different concentrations of [3H]-CP 55,940. Specific binding of [
3H]-CP
55,940 was calculated from the difference between total and non-specific binding ············· 108
Figure 4.5 Displacement curves of the [3H]-CP 55,940 binding with WIN as a competitive
inhibitor and the IC50 values of WIN at 1 day, 3 days, and 7 days following sham or BVD
surgery ······························································································································· 109
APPENDIX 1: THE SPECIFICITY OF CB1 RECEPTOR LABELLING IN THE RAT HIPPOCAMPUS
Table 1.1 Various combinations of CB1 receptor primary and subsequent secondary antibody

List of Figures and Tables xviii
titres trialled and their incubation durations ········································································ 129
Figure 1.1 CB1 receptor labelling with different primary antibody incubation durations ···· 131
Figure 1.2 CB1 receptor labelling with different primary antibody concentrations·············· 132
Figure 1.3 CB1 receptor labelling with different secondary antibody incubation durations · 133
Figure 1.4 CB1 receptor labelling with different secondary antibody concentrations ·········· 134
Figure 1.5 Double labelling immunofluorescence of the CB1 receptor antibody with an
antibody for NeuN, in the hippocampus ············································································· 135
Figure 1.6 Immunohistochemical labelling of the CB1 receptor in axons and dendrites in the
stratum oriens and DG regions of the hippocampus ···························································· 136
APPENDIX 2: CHANGES IN CB1 RECEPTOR EXPRESSION FOLLOWING BILATERAL
VESTIBULAR DEAFFERENTATION IN RATS
Figure 2.1 Representative immunohistochemical sections of the CB1 receptor labelling in the
hippocampus of a sham and a BVD animal ········································································ 143
Figure 2.2 The spatial distribution of the CB1 receptors across the CA1, CA3, and DG of the
hippocampus at 1, 3, and 7 days following BVD compared to the sham controls ················ 144
Figure 2.3 The AUC of the spatial distribution of CB1 receptors in the dorsal and ventral
hippocampus at 1, 3, and 7 days following BVD compared to the sham controls ················ 145
APPENDIX 3: THE SPECIFICITY OF CB2 RECEPTOR LABELLING IN THE RAT HIPPOCAMPUS
Figure 3.1 A CB2 receptor diagram showing the locations of different commercial and
privately made CB2 receptor primary antibodies targeting different epitopes of the CB2
receptor protein ·················································································································· 150
Table 3.1 Various combinations of CB2 receptor primary (1o) and subsequent secondary (2
o)
antibody titres trialled and their incubation durations ························································· 152
Figure 3.2 Western blots of hippocampal tissue (Hp1 and Hp2) and spleen (sp, as a positive

List of Figures and Tables xix
control), using the Santa Cruz and Abcam CB2 receptor antibodies ···································· 154
Figure 3.3 Immunohistochemical comparison of CB2 receptor labelling with different
primary antibody concentrations in the dentate gyrus (DG) of the rat hippocampus ············ 155
Figure 3.4 Immunohistochemical comparison of CB2 receptor labelling with different
primary antibody concentrations in the DG of the rat hippocampus ···································· 156
Figure 3.5 Immunohistochemical comparison of CB2 receptor labelling with different
secondary antibody concentrations in the DG of the rat hippocampus································· 156
Figure 3.6 Immunohistochemical comparison of CB2 receptor labelling with different
antibody incubation durations in the DG of the rat hippocampus ········································ 157
Figure 3.7 Immunohistochemical labelling of CB2 receptors in the hippocampus ·············· 158
Figure 3.8 Double labelling immunofluorescence of the CB2 receptor antibody with a NeuN
antibody, a neuronal marker in various regions of the hippocampus ··································· 159
Figure 3.9 Immunofluorescent labelling showing the distribution of the CB2 receptors in the
CA3 region of the hippocampus using antibodies from the Abcam (rabbit polyclonal) and
Santa Cruz (goat polyclonal) ······························································································ 160

List of Abbreviations xx
LIST OF ABBREVIATIONS
Abbreviation Explanation
2-AG 2-arachidonyl glycerol
ADN Anterodorsal nucleus
AEA Anandamide
AMPA -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
ANOVA Analysis of variance
APS Ammonium persulfate
AUC Area under the curve
BSA Bovine serum albumin
BVD Bilateral vestibular deafferentation
CA1 Cornu Ammonis area 1
CA3 Cornu Ammonis area 3
cAMP Cyclic adenosinemonophosphate
CB1 receptor Cannabinoid 1 receptor
CB2 receptor Cannabinoid 2 receptor
cDNA Complimentary deoxyribonucleic acid
CNS Central nervous system
CT Computed tomography
DAB Diaminobenzidine
DAG Diacylglycerol
DAGL Diacylglycerol lipase
DG Dentate gyrus
dH2O Distilled water
DMS Delayed matching to sample
DMSO Dimethyl sulfoxide
DNA Deoxyribonucleic acid
DNMS Delayed nonmatch to sample
DSE Depolarisation-induced suppression of excitation
DSI Depolarisation-induced suppression of inhibition
DTN Dorsal tegmental nucleus
EC Entorhinal cortex

List of Abbreviations xxi
EEG Electroencephalography
EMT Endocannabinoid membrane transporter
FAAH Fatty acid amide hydrolase
GABA -aminobutyric acid
GLM General linear model
GPCR G-protein coupled receptor
HRP Horse radish peroxidase
IAC Internal auditory canal
IC50 Half maximal inhibitory concentration
IPSC Inhibitory postsynaptic current
LMM Linear mixed model
LMN Lateral mammillary nucleus
LTD Long-term depression
LTP Long-term potentiation
MAGL Monoacylglycerol lipase
mGluR Metabotropic glutamate receptor
MRI Magnetic resonance imaging
NADA N-arachidonoyl-dopamine
NAPE-PLD N-acyl phosphatidyl ethanolamine phospholipase D
NArPE N-arachidonoyl-phosphtidyl ethanolamine
NF-L Neurofilament-L
NMDA N-methyl-D-aspartate
NOS Nitric oxide synthase
PLC Phospholipase C
PPTN Pedunculopontine tegmental nucleus
PS Post-subiculum
RNA Ribonucleic acid
SDS Sodium dodecylsulfate
SNAP-25 Synaptosome-associated protein of 25 kDa
SOR Spontaneous object recognition
SUM Supramammillary nucleus
TEMED N,N,N‟,N‟-tetremethylethylenediamine
UVD Unilateral vestibular deafferentation
VCR Vestibulocollic reflex
VNC Vestibular nucleus complex

List of Abbreviations xxii
VOR Vestibulo-ocular reflex
VSR Vestibulospinal reflex
WIN WIN 55212-2
o C Degrees Celsius
9-THC Delta-9-tetrahydrocannabinol

1
Chapter 1
Introduction

Chapter 1: Introduction 2
The vestibular system in the inner ear is the sensory system primarily responsible for
sensing self-motion and for stabilising the eyes and the body. It has been reported that damage
to the vestibular system leads to debilitating psychological and emotional problems as well as
cognitive impairments in humans and animals. Furthermore, significant synaptic changes
occur following vestibular damage in the brain areas that are involved in processing vestibular
information. As it has been demonstrated that the endocannabinoid system is critically
involved in synaptic plasticity and in cognitive function, this thesis examines the role of the
endocannabinoid system in the brain following vestibular damage, and investigates neural
mechanisms that may underlie the debilitating effects of vestibular damage (Figure 1.1).
Figure 1.1
Venn diagram showing the possible association between the vestibular and endocannabinoid
systems, together with their discrete involvement in cognitive function and synaptic plasticity.
Synaptic Plasticity
&
Cognitive Function
Endocannabinoid
System
Vestibular
System
?

Chapter 1: Introduction 3
1.1 The Vestibular System
Unlike other sensory systems, the functions and sensations of the vestibular system are
not prominent, readily recognisable, or localisable in our consciousness. However, the
influence of the vestibular system is crucial for almost every aspect of human daily activities
and „normal‟ behaviour. The physiological significance of this “hidden” sensory system was
uncovered by early animal experimental pioneers such as Flourens, Goltz, Stefani, Breuer,
Mach, von Cyon, Ewald, Magnus, Ménière, and Bárány. They demonstrated that surgical
ablation and mechanical stimulation of the vestibular apparatus led to a dramatic and often
peculiar disorganisation of posture and movement (Choi et al., 2007; Heskin-Sweezie et al.,
2010; Parietti-Winkler et al., 2010; see Horak, 2009 for review).
Stimulation of the vestibular system can be achieved by linear or angular accelerations
of the whole body. The vestibular system receives information about the movement and the
position of the head in space, in order to maintain head and body posture (Peterson and
Richmond 1988) and to stabilise gaze during walking and running (Grossman et al., 1988,
1989). Head movements in space are often a complex combination of angular rotation and
linear translation. Nevertheless, the vestibular system has evolved to sense changes in angular
and linear velocity in all three dimensions, and allows the brain to receive exact information
about head movement and position even without visual input in most organisms, including in
humans (see Angelaki and Cullen, 2008; Angelaki et al., 2009 for reviews).
1.1.1 Anatomical Structure and Function
In order to identify anomalies under conditions of vestibular dysfunction, it is necessary
to understand the normal structure and function of the vestibular system. The vestibular
system is composed of three components: a peripheral sensory apparatus, a central processor,
and a mechanism for motor output. The peripheral apparatus consists of a set of acceleration
sensors that together are known as the vestibular labyrinth, which sends information to the
central nervous system (CNS) about head angular and linear acceleration, and orientation of
the head with respect to gravity. The major role of the peripheral vestibular apparatus is to
signal the brain about the acceleration of the head or changes in gravitational forces, so that
related muscle groups are activated to produce movements of the body and eyes to allow the
organism to adapt to maintain appropriate posture and balance (see Cohen and Keshner, 1989;
Keshner and Cohen, 1989 for reviews).

Chapter 1: Introduction 4
The vestibular labyrinth is located lateral and posterior to the cochlea in the inner ear,
and is contained in the petrous portion of the temporal bone. The peripheral vestibular system
has two different types of detectors: the semicircular canals and the otoliths (Figure 1.2). The
three semicircular canals (the horizontal, anterior, and posterior canals), detect primarily
angular acceleration of the head in space and the two otolith organs (the utricle and the
saccule), have a unique role in registering changes in gravitational force or linear acceleration
by gravity (see Kondrachuk, 2001 for review).
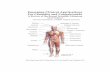
Figure 1.2
A schematic representation of the structure of the vestibular labyrinth (purple) with its
innervating vestibular and cochlear nerves (orange). The illustration has been modified from
Glover (2009), with permission from Elsevier.
It is often assumed that the three semicircular canals lie at right angles to one another
and that the left and right sets of canals represent true mirror images of each other on either
side of the sagittal plane. However, a series of anatomical studies by Markham and colleagues
has shown that neither of these assumptions is true (Blanks et al., 1972, 1975; Curthoys et al.,
1975, 1977). Furthermore, with the advances in high-resolution computed tomography (CT)
and magnetic resonance imaging (MRI), it has become possible to obtain accurate knowledge
of the absolute orientation and position of the semicircular canals (Della Santina et al., 2005;
Lane et al., 2008; Bradshaw et al., 2010). When the horizontal canal in humans is positioned
so that it is parallel to the earth‟s horizontal, the anterior canal is positioned at an angle of
approximately 110 degrees with respect to the horizontal canal while the posterior canal is at
an angle of approximately 95 degrees. In addition, the angles between semicircular canals

Chapter 1: Introduction 5
vary between individuals and species (see Wilson and Melvill Jones, 1979).
Each semicircular canal is comprised of a bony and membranous duct. Within the bony
labyrinth, membranous ducts and sacs are separated from the bony part by a fluid called
perilymph. In all three canals, the membranous duct opens into a sac known as the utricle, and
each canal together with the utricle forms a closed ring in which the second fluid, called
endolymph, circulates. Each semicircular canal is widened at one end to form an ampulla,
where the hair cells and their supporting cells are located in a crista. Angular acceleration in
the plane of the canal creates inertial force that acts on the endolymph circulation around the
canal, which in turn induces physical displacement of the stereocilia of the hair cells in the
same direction, stimulating the hair cells. As the membranous labyrinths are anchored within
the otic capsule, the displacement of the hair cells accurately corresponds to the acceleration
of the skull at all times (see Wilson and Melvill Jones, 1979).
The macula of the two otolith organs is a structure analogous to the crista in the
semicircular canals. The utricular macula is horizontally oriented along the base of the utricle,
and is primarily responsible for transduction of earth-horizontal linear acceleration in any
direction, and registers static tilt with respect to gravity. Beneath the utricle lies the saccule,
containing the saccular macula. This component has a similar role to that of the utricular
macula, but is arranged in an approximately orthogonal plane on the medial wall of the
saccule to detect acceleration in the vertical plane (Buttner-Ennever, 1999; Curthoys et al.,
2009).
Hair cells in the otolith organs are organised in a similar way to the ampulla except for
the presence of calcium carbonate crystals, the otoconia, above the otolithic membrane. In the
utricle and saccule, the otolithic membrane covers the stereocilia of the hair cells. The relative
displacement of the otoconia, by linear acceleration or the gravity-induced inertial force
acting in the plane of the macula, causes the stereocilia to bend. This mechanical deflection of
the cilia stimulates the sensory hair cells, which in turn generates trains of action potentials in
the primary afferent nerve fibres (see Wilson and Melvill Jones, 1979).
There are two morphologically different types of hair cells in the vestibular sensory
epithelium (Figure 1.3). The type I cells are flask-shaped and are surrounded by a single large
primary afferent nerve terminal in the form of a nerve chalice. The type II cells are more
cylindrically shaped, and are innervated by a series of bouton-type nerve terminals at their

Chapter 1: Introduction 6
base. These sensory cells are called hair cells due to the stereocilia that project from the apical
surface of the cell. They are found in the cristae at the ampullary end of the semicircular
canals, and in the maculae of the utricle and saccule (see Zajonc and Roland, 2005). The
bundle of stereocilia found on the top of these hair cells is arranged in a highly systematic
manner. The stereocilia are arranged in rows of increasing height, where the tallest
stereocilium lies next to a single cilium of a different kind, the kinocilium (Figure 1.3). The
circulation of the endolymph in the semicircular canals, and the otoconial movement in the
case of the maculae, causes the mechanical bending of the stereocilia of the hair cells, which
in turn is translated into a graded electrical potential. These graded electrical potentials in the
hair cells are then translated into action potentials that propagate down the afferent nerves
towards the vestibular nuclei (see Wilson and Melvill Jones, 1979).
Figure 1.3
A schematic representation of the structure and organisation of different types of hair cells in
the vestibular sensory epithelium. The illustration has been adapted from Meyers et al. (2009),
with permission from Elsevier.
The direction of this mechanical deflection, either towards or away from the kinocilium,
is associated with excitation or suppression of primary afferent neural activity, respectively.
Deflection of the stereocilia towards the kinocilium causes the hair cells to depolarise, which
in turn increases the rate of firing in the vestibular afferent fibres. In contrast, if the stereocilia

Chapter 1: Introduction 7
are bent away from the kinocilium, the hair cell becomes hyperpolarised, subsequently
reducing the afferent firing rate (Loewenstein, 1974; Goldberg and Hudspeth, 2000). Given
that the opposing physiologic responses arise from mechanical deflections of the stereocilia,
the hair cells in the vestibular organs are arranged in a specific orientation in order to signal
the precise direction of the angular or linear acceleration. In the semicircular canals, the
ampullary hair cells of the complementary pairs of canals are arranged in such a way that the
depolarisation of the hair cells occurs in opposite directions in response to any component
rotational acceleration in the plane of those canals. Therefore, the three semicircular canals,
lying approximately orthogonal to one another, are able to respond accurately to any
rotational movement made in three-dimensional space. In the two otolith organs, each of the
utricular and saccular maculae is divided into two areas by curved lines, the striolae. The hair
cells are polarised in opposite directions on either side of the striolae, which effectively
separate the receptors into two morphologically opposed groups. Due to the curvature of the
striolae, the otolithic hair cells are able to respond sensitively to head positions and linear
accelerations in all directions (Wilson and Melvill Jones, 1979).
1.1.2 Vestibular Pathways
The peripheral vestibular nerve fibres that arise from the receptor hair cells in the
semicircular canals and the otolith organs converge as they course toward Scarpa‟s (vestibular)
ganglion, which contains the bipolar ganglion cell bodies of the afferent nerve fibres within
the bony internal auditory canal (IAC). In addition to the vestibular nerve, the IAC contains
the cochlear nerve, the facial nerve, the nervus intermedius (a branch of the facial nerve), and
the labyrinthine artery (see Wilson and Melvill Jones, 1979; Figure 1.2). Inputs from the
primary vestibular afferents reach two main areas in the brain: the vestibular nucleus complex
(VNC) and the cerebellum (Carleton and Carpenter, 1984). The VNC is located along the
lateral wall of the fourth ventricle between the roof of the brainstem and the floor of the
cerebellum. The VNC is divided into four “major” nuclear groups; superior, medial, lateral,
and descending (inferior), as well as some smaller cell groups (Brodal and Pompeiano, 1957;
Brodal, 1974). However, the boundaries of the specific vestibular nuclei are difficult to
distinguish based on their cytological characteristics (see Barmack, 2003; Highstein and
Holstein, 2006 for reviews). Once head motion signals are received by the VNC, various
subnuclei (the superior, medial, lateral, descending vestibular nuclei and the prepositus
hypoglossi nucleus), process specific types of angular and linear velocity information. This
process is highly ordered. Both of the superior and medial vestibular nuclei are responsible for

Chapter 1: Introduction 8
the vestibulo-ocular reflexes (VORs), and the lateral vestibular nucleus is the nucleus
primarily responsible for the vestibulo-spinal reflexes (VSRs). However, the medial vestibular
nucleus is also involved in the VSRs. The descending nucleus communicates with all of the
other nuclei, yet does not have a primary output of its own. Primary vestibular fibres do not
cross the midline of the brainstem, yet the labyrinth on one side greatly influences the activity
of the VNC of the other. This marked effect is achieved through the commissural fibres that
interconnect the VNC of the two sides (Hain and Helminski, 2007). In the VNC, processing of
vestibular sensory inputs occurs simultaneously with the processing of other sensory
information such as proprioceptive, visual, tactile, and auditory inputs (see Smith et al., 2005a
for review).
The secondary vestibular fibres from the VNC provide an ascending input to cranial
motor nuclei III, IV and VI, controlling the reciprocal contractions of extraocular muscles to
make reflexive eye movements (i.e. VORs) to compensate for head movement (Figure 1.4).
Outputs of the VNC not only evoke reflexes mediated by extraocular muscles, they also evoke
skeletal muscle reflexes. Descending projections of the VNC to spinal motor centres originate
from the lateral, medial, and descending vestibular nuclei (Brodal, 1981) to form lateral and
medial vestibulospinal tracts. Some of the vestibular information is also relayed by
reticulospinal tracts, which originate in the pontomedullary reticular formation (Wilson and
Melville Jones, 1979). These projections allow for the generation of a variety of reflexes of
the body and limb musculature to control body posture and maintain balance (i.e. VSR)
during head motion and against gravity (Figure 1.4). Some of the vestibular information from
the VNC is also relayed onto the cerebellum (see Highstein and Holstein, 2006 for review;
Figure 1.4). Although the cerebellum is not required for vestibular reflexes, vestibular reflexes
cannot be regulated or function effectively when this structure is absent (Beraneck et al., 2008;
Cullen et al., 2009; see Mackay and Murphy, 1979 for review). Conscious awareness of self-
motion in darkness relies on ascending pathways from the VNC to the thalamus, from which
vestibular information is projected into many areas of the neorcortex and limbic system (see
Smith et al., 2005a for review).

Chapter 1: Introduction 9
Vestibular input
Non-vestibular
sensory input
VNC
Cranial motor nuclei
Cerebellum
Spinal motor centre
VOR
VSR
Figure 1.4
Simplified diagram representing the organisation of the key areas and pathways for the
production of the VORs and VSRs. Abbreviations: VNC, vestibular nucleus complex; VOR,
vestibulo-ocular reflex; VSR, vestibulo-spinal reflex.
1.1.3 Peripheral Vestibular Deafferentation
As the brain processes vestibular signals by comparing those received from both ears, it
is critical that both vestibular labyrinths are intact in order to achieve normal vestibular
reflexes. However, should a loss of vestibular function occur, vestibular reflex activity is
disturbed, resulting in a syndrome of ocular motor and postural disorders due to the
interruption of central vestibulo-ocular and vestibulo-spinal pathways.
Much of our understanding of vestibular function is due to accumulated clinical and
experimental observations in humans and animals with unilateral and bilateral vestibular
lesions. The effects of unilateral or bilateral vestibular deafferentation (UVD and BVD,
respectively) have been studied extensively in animals (Smith et al., 1986; Smith and
Curthoys, 1988a, b; Zennou-Azogui et al., 1993; Hamann et al., 1998; Newlands et al., 2005;
Goddard et al., 2008a; Zheng et al., 2004, 2006, 2007, 2009b) and in humans (Honrubia et al,
1984, 1985; Fetter et al., 1991; Brandt et al., 2005; Hüfner et al., 2007; Lopez et al., 2007;
Mbongo et al., 2009). The symptoms that occur following UVD can be categorised into two
groups: static or dynamic. Static symptoms include spontaneous ocular nystagmus (quick
phases towards the intact side), yaw head tilt and, roll head tilt, and dynamic symptoms
include abnormal amplitude (gain) and timing (phase) of the VORs and VSRs in response to
head movement (see Darlington and Smith, 2000 for review).
Following UVD, the spontaneous resting activity of neurons in the VNC ipsilateral to
the lesion decreases, while it increases on the contralateral side (see Smith and Curthoys,
1989 for review). As a result of this imbalance in spontaneous resting activity between
neurons in the ipsilateral and contralateral VNCs, asymmetrical activation of the vestibular

Chapter 1: Introduction 10
reflexes causes a dramatic series of symptoms such as spontaneous nystagmus, postural
instability, and inadequate compensatory responses to head movement (see Smith and
Curthoys, 1989 for review).
In humans, a sudden unilateral loss of labyrinthine function can result from accidents,
ototoxicity, diseases, or from surgical treatment for diseases such as Ménière‟s disease. The
consequence of a sudden loss of unilateral labyrinthine function is dramatic, and patients
suffer from severe dizziness, nausea and vomiting (Baloh, 1979). Although UVD patients
show “normal” upright stance, provided that at least one accurate sensory input (visual or
somatosensory) is present, severe postural disturbances are observed when UVD patients are
forced to rely solely on vestibular afferent input (Nashner et al., 1982; Horak et al., 1990;
Fetter et al., 1991; Maurer et al., 2000; Mergner et al., 2009). The severe ocular and postural
deficits due to UVD, however, are temporary and are partially reduced by a recovery process
known as „vestibular compensation‟ in both animals (Norre et al., 1984; Sirkin et al., 1984;
Smith et al., 1986; Newlands and Perachio, 1990; Gilchrist et al., 1998; Heskin-Sweezie et al.,
2010) and humans (Fetter et al., 1991; Choi et al., 2007; Parietti-Winkler et al., 2010; see
Horak, 2009; Saman et al. 2009 for review). The vestibular compensation process is thought
to involve synaptic plasticity within the VNC (zu Eulenburg et al., 2010; see Dieringer, 1995;
Darlington et al., 2002; Olabi et al., 2009 for reviews). During vestibular compensation,
altered resting activity in each of the two VNCs returns to near normal, and as it does so,
some of the symptoms of UVD decrease (Yamanaka et al., 2000; Him and Dutia, 2001;
Bergquist et al., 2008; Helmchen et al., 2009; Heskin-Sweezie et al., 2010; see Lacour and
Tighilet, 2010 for review). However, the compensation process is never complete. Although
many of the static symptoms due to UVD diminish within a few days to a week, the
symptoms related to the deficits in dynamic function (i.e. gain and phase) of the vestibular
reflexes remain (Fetter et al., 1996; Hamann et al., 1998; Gilchrist et al., 1998; Lopez et al.,
2007; Heskin-Sweezie et al., 2010; Parietti-Winkler et al., 2010; see Dutia, 2010 for review).
The effects of BVD are different to UVD. The static symptoms of UVD do not develop
following BVD due to an equal reduction in the resting activity in both VNCs (Ris and
Godaux, 1998; see Smith and Curthoys, 1989 for review). However, since BVD results in
complete loss of the VORs and VSRs, severe oscillopsia (a blurring of the vision during head
movement) and ataxia (uncoordinated muscle movements) occur as a result (see Brandt,
1996). Following BVD, despite the regeneration of spontaneous resting activity in the VNC
neurons, the dynamic sensitivity to head movement never recovers (Ryu and McCabe, 1976;

Chapter 1: Introduction 11
Waespe et al,. 1992; Yakushin et al., 2005). Therefore, there is no recovery in VOR function
following BVD, although there is some evidence to suggest that some compensation may
occur by substituting smooth pursuit eye movements for the absent VORs (Bense et al., 2004;
Bockisch et al., 2004; Dieterich et al., 2007; Herdman et al., 2007). While ataxia gradually
decreases over time (Igarashi and Guitiierrez, 1983; Igarashi et al., 1988), some residual VSR
symptoms such as high-acceleration postural perturbation are observed (Allum and Pfaltz,
1985; Horak et al., 1990).
Interestingly, BVD results in hyperactivity in rats while reducing exploratory behaviour
and thigmotaxis (bodily wall contact during movement) (Goddard et al., 2008a). Furthermore,
BVD has been shown to cause emotional changes not only in humans (Furman and Jacob,
2001; Staab and Ruckenstein, 2003; Sang et al., 2006; Jáuregui-Renaud et al., 2008; Kalueff
et al., 2008), but also in animals (Goddard et al., 2008a; Zheng et al., 2008). It is clear that
following UVD or BVD, the processing of visual, auditory, and proprioceptive sensory
information is disrupted. These disturbances cause significant changes in neural activity in the
VNC, which may result in changes further upstream in the processing networks of the brain.
Furthermore, vestibular damage also results in cognitive deficits in animals (Wallace et al.,
2002b; Russell et al., 2003a; Zheng et al., 2004, 2006, 2007, 2009b) and in humans
(Schautzer et al., 2003; Brandt et al., 2005), which are thought to be related to vestibular
input to the hippocampal region of the brain.
1.1.4 Vestibular-Hippocampal Connections
A great deal of research on learning and memory has focused on the hippocampus, a
brain structure implicated in these processes (see Olton and Papas, 1979; Squire, 1992; Rolls,
1996; Burgess et al., 2002; Squire et al., 2004 for reviews). Furthermore, a considerable
amount of data suggests the importance of the hippocampus for the construction and retrieval
of spatial memory maps and the ability to navigate through environments (Morris et al., 1982;
Jarrard 1993; Burgess et al., 2002; Yim et al., 2008; de Oliveira Alvares et al., 2008), with a
number of studies showing impairments in spatial tasks following hippocampal damage for
both animals (Whishaw and Maaswinkel, 1998; Whishaw and Gorny, 1999; Gilbert and
Kesner, 2002; Broadbent et al., 2004; Talpos et al., 2008; Faraji et al., 2008; Iordanova et al.,
2009) and humans (Altemus and Almli, 1997; Kessels et al., 2001; Spiers et al., 2001; Astur
et al., 2002).

Chapter 1: Introduction 12
Interest in the possibility of a connection between the vestibular system and the
hippocampus has been provided by previous studies in both experimental animals and human
patients showing that the vestibular system contributes to the development of spatial memory
(Etienne, 1980; Matthews et al., 1989; Israel et al., 1996; Cuthbert et al., 2000; Russell et al.,
2003a, 2006; McGauran et al., 2005; Allen et al., 2007; Nardini et al., 2008).
Spatial memory deficits in BVD animals have been demonstrated in numerous
behavioural tasks such as the radial arm maze (Matthews et al., 1989; Ossenkopp and
Hargreaves, 1993; Russell et al., 2003a), the spatial forced-alternation T-maze (Zheng et al.,
2007), and the foraging task (Wallace et al., 2002c; Zheng et al., 2006, 2009b). In a more
recent study by Zheng et al. (2009b), it was shown that although rats with BVD have been
found to be able to use visual cues to navigate themselves home under light conditions, their
ability to perform in the foraging task was severely impaired in darkness when compared with
sham rats (Zheng et al., 2009b). Although UVD animals have been found to show spatial
memory deficits at 3 months post-operation in performing the foraging task, recovery from
these deficits has been observed by 6 months post-operation (Zheng et al., 2006).
In parallel with animal studies, impairments in the spatial memory and navigational
abilities of humans with vestibular dysfunction have been shown to endure for up to 10 years
following the loss of vestibular function (Grimm et al., 1989; Risey and Briner, 1990; Peruch
et al., 1999; Schautzer et al., 2003; Redfern et al., 2004; Brandt et al., 2005; see Smith et al.,
2005b; Hanes and McCollum, 2006 for reviews). A landmark study was published by Brandt
and colleagues in 2005 in which the volume of the hippocampus and performance in spatial
memory tests in patients with acquired chronic bilateral vestibular loss were compared with
age-, sex-, and education-matched controls (Brandt et al., 2005). Intriguingly, patients with
bilateral vestibular loss exhibited significant impairment in performing in spatial memory and
navigation tasks when tested with a virtual Morris water maze. Moreover, MRI scans of these
patients revealed a selective and bilateral atrophy of the hippocampus, suggesting that spatial
memory and navigation rely on vestibular input (Brandt et al., 2005). Interestingly, Hüfner et
al., (2007) showed that while patients with left vestibular loss showed no significant deficits
in spatial memory and navigation, patients with right vestibular loss showed a greater heading
error compared to left UVD patients or to control subjects in the probe trial in a virtual Morris
water maze. Furthermore, hippocampal atrophy was not observed in patients with unilateral
vestibular loss whether the vestibular loss was due to acoustic neurinoma (Hüfner et al., 2007)
or vestibular schwannoma removal (Hüfner et al., 2009). However, more recently, zu

Chapter 1: Introduction 13
Eulenberg and colleagues demonstrated that patients with unilateral vestibular loss due to
vestibular neuritis exhibited an atrophy of the left posterior hippocampus (zu Eulenberg et al.,
2010). The authors postulated that this atrophy may be due to a reduced inflow of vestibular
input after an incident of vestibular neuritis (zu Eulenberg et al., 2010). Interestingly,
structural and functional plasticity of the hippocampus in healthy humans has also been
demonstrated. For example, London taxi drivers (Maguire et al., 2000), professional dancers
and slackliners (Hüfner et al., 2010) have all shown a decrease in anterior hippocampal
volume with an increase in posterior hippocampal volume compared to age- and sex-matched
controls. Taken together, these results emphasise the role of the vestibular system in spatial
memory function, and outline the importance of vestibular input to the hippocampus for
maintaining its integrity.
A number of anatomical connections between the VNC and the hippocampus have been
suggested. Some vestibular information reaching the hippocampus passes through the
thalamus, the parietal cortex, and the entorhinal or perirhinal cortex and then to the
hippocampus (Figure 1.5). However, more direct connections between the VNC and the
hippocampus are also possible. These include a theta-generating pathway leading from the
pedunculopontine tegmental nucleus via the supramammillary nucleus and medial septum to
the hippocampus, or the head direction system passing through the dorsal tegmental nucleus,
lateral mammillary nucleus, anterodorsal thalamic nucleus, and the post-subiculum to the
hippocampus (see Smith, 1997; Smith et al., 2005a for reviews; Figure 1.5).

Chapter 1: Introduction 14
Vestibular Nucleus
Thalamus
Parietal cortex
Entorhinal/Perihinal
cortices
Hippocampus
PPTN
SUM
Medial
septum
DTN
LMN
ADN
PS
Figure 1.5
Some potential vestibulo-hippocampal connections. Colour codes for the pathways are:
thalamo-cortical route; theta-generating route; and head-direction route. Abbreviations: DTN,
dorsal tegmental nucleus; PPTN, pedunculopontine tegmental nucleus; SUM, supramammillary
nucleus; LMN, lateral mammillary nucleus; ADN, anterodorsal nucleus; PS, post-subiculum.
Many studies have reported evidence for vestibular-hippocampal connections through
the use of neurochemical, electrophysiological, and imaging methods. The first neurochemical
evidence to support the link between the vestibular system and the hippocampus was provided
by Horii et al. (1994). In this study, electrical stimulation of the vestibular nerve caused a
large increase in acetylcholine release in the hippocampus, which was inhibited by the
administration of a non-NMDA (N-methyl-D-aspartate) receptor antagonist into the VNC
(Horii et al., 1994). Although the authors postulated that an increase in histamine release in
the medial septum following the electrical stimulation of vestibular nerve (Mochizuki et al.,
1994) might affect acetylcholine release in the hippocampus, depletion of neuronal histamine
did not prevent the increased release of acetylcholine in the hippocampus (Horii et al., 1995,
1996). Since then, numerous studies have demonstrated neurochemical changes in the
hippocampus following peripheral vestibular damage, providing compelling evidence for
neural connections between the vestibular system and the hippocampus (Table 1.1). In
particular, in a study by Ashton et al. (2004b), although there was no change in cannabinoid
CB1 receptor expression in the hippocampus following UVD in rats, this was the only study

Chapter 1: Introduction 15
that investigated possible changes in the endocannabinoid system following vestibular
damage. However, it must be remembered that the Ashton et al. (2004b) study was carried out
in UVD animals and given that UVD and BVD result in quite different symptoms, no change
in the CB1 receptors following UVD does not necessarily imply that there would be no
change following BVD.
Evidence for a connection between the vestibular system and the hippocampus has been
further provided by studies showing that vestibular stimulation leads to activation of the
hippocampus in animals (O‟Mara et al., 1994; Gavrilov et al., 1995, 1996; Sharp et al., 1995;
Cuthbert et al., 2000; Hicks et al., 2004; Horii et al., 2004) and in humans (Vitte et al., 1996;
Suzuki et al., 2001; Dieterich et al., 2003). In particular, Cuthbert et al. (2000) demonstrated
that high frequency electrical stimulation in one vestibular labyrinth of guinea pigs evoked
field potentials in the hippocampus bilaterally with a latency of approximately 40
milliseconds. The electrical stimulation used in this study was below the threshold to produce
eye movements, suggesting that the hippocampal responses were not confounded by feedback
from the eye muscles or from efferent copy signals (Cuthbert et al., 2000). Similar findings
were reported by Hicks and others who also showed that these hippocampal responses can be
elicited from multiple areas of the guinea pig vestibular labyrinth including the canal
ampullae, utricle and saccule (Hicks et al., 2004).

Chapter 1: Introduction 16
Table 1.1
Neurochemical changes in the hippocampus and in the neocortex following vestibular damage.
Neural Substrate Changes in Level of Expression Reference
UVD
eNOS
↓ in the contralateral CA2/3
↑ in the contralateral DG Liu et al., 2003c
↑ in the contralateral EC and PRC Liu et al., 2004
nNOS ↓ in the ipsilateral DG
Zheng et al., 2001;
Liu et al., 2003c
↓ in the contralateral EC and PRC Liu et al., 2004
Arginase I ↑ in the contralateral PRC
↓ in the ipsilateral PRC Liu et al., 2004
Arginase II No change in the hippocampus Liu et al., 2003c
↑ in the ipsilateral EC Liu et al., 2004
NMDA receptor s
subunits
↓ in the ipsilateral CA2/3 (NR1)
Liu et al., 2003b ↓ in the ipsi- and contralateral CA2/3
(NR2A)
↑ in the ipsilateral CA1 (NR2A)
CB1 receptors No change in the hippocampus Ashton et al., 2004b
Glucocorticoid
rereceptors No change in the hippocampus Lindsay et al., 2005
BVD
SNAP-25 ↑ in the DG
Goddard et al., 2008c Synaptophysin
No change in the hippocampus, EC,
PRC, FC Drebrin
NF-L
Serotonin
tratransporter ↓ in the FL and CA1
Goddard et al., 2008b
Tryptophan
hyhydroxylase ↑ in the FL, CA2/3, and DG
Dopamine--
hyhydroxylase
No change in the hippocampus, EC,
PRC, FL
Dopamine
tratransporter
No change in the hippocampus, EC,
PRC, FL
Tyrosine
hyhydroxylase
No change in the hippocampus, EC,
PRC, FL
Abbreviations: DG, dentate gyrus; EC, entorhinal cortex; eNOS, endothelial nitric oxide synthase; FL,
frontal lobes; nNOS, neuronal nitric oxide synthase; NF-L, neurofilament-L; NMDA, N-methyl-D-aspartate;
PRC, perirhinal cortex; SNAP-25, synaptosome-associated protein of 25 kDa.

Chapter 1: Introduction 17
Under normal conditions, hippocampal place cells fire when an animal moves through a
specific location in an environment (O‟Keefe and Dostrovsky, 1971), and enable animals to
construct their location within the environment (O‟Keefe and Nadel, 1978). The spatial-
specific firing pattern of the hippocampal place cells is maintained even in the absence of
visual input (Hill and Best, 1981; Quirk et al., 1990; Save et al., 1998), and these
hippocampal place cells are thought to have an essential role in spatial navigation (Knierim et
al., 1995; Wiener et al., 1995; see O‟Keefe, 1979 for review). Interestingly, the activity of
these place cells has been shown to be influenced by electrical stimulation of the VNC. For
example, Horii et al. (2004) showed that the firing rates of hippocampal CA1 neurons were
altered following the electrical stimulation of the medial vestibular nucleus in rats using single
unit recording. The firing rates of the place cells in the hippocampus (i.e. complex spike cells)
were increased in a current intensity-dependent manner, while only some of the theta cells (i.e.
non-complex spiking cells) responded to vestibular stimulation (Horii et al., 2004).
Following bilateral inactivation of the vestibular system, whether the damage is
temporary (e.g. transtympanic injection of tetrodotoxin; Stackman et al., 2002) or permanent
(surgical lesions of the labyrinth; Russell et al., 2003b, 2006), the response of place cells and
theta activity in the CA1 region of the hippocampus has been shown to be disrupted.
Specifically, Stackman and colleagues showed that tetrodotoxin-induced vestibular
inactivation disrupted the location-specific firing of hippocampal place cells and disintegrated
the place fields. Furthermore, place cell spatial coherence and spatial information content
were significantly decreased, while place cell peak or average firing rate was unaffected
(Stackman et al., 2002). Spatial coherence is a measure of the orderliness of discharge within
and outside the place fields (Kubie et al., 1990) and spatial information content is a
quantitative measure of the amount of information conveyed by each spike (Skaggs et al.,
1993). Russell et al. (2003b) reported that the firing fields of place cells in BVD animals were
larger compared to sham animals, indicating that the location-specific firing of place cells in
BVD animals was disturbed. The place cells of BVD animals had lower spatial coherence and
information content, while the average firing rate, but not the peak firing rate, was higher
(Russell et al., 2003b). In addition, a larger field size and average firing rate in theta cells was
observed in BVD animals (Russell et al., 2003b), but Stackman et al. (2002) did not find any
changes in theta activity. Nevertheless, a decrease in the rhythmicity of theta activity in the
hippocampus has been observed in BVD rats (Russell et al., 2006).
In an attempt to further clarify the mechanism underlying the dysfunction of

Chapter 1: Introduction 18
hippocampal place cells following vestibular damage, Zheng and colleagues carried out in
vitro electrophysiological studies involving hippocampal brain slices from UVD rats. In this
study, there was a significant reduction in field potential responses to electrical stimulation in
the Schaffer collaterals in the CA1 area at 5-6 months following the lesion, indicating a
significant loss of normal electrical excitability (Zheng et al., 2003). Although more profound
effects on hippocampal electrical excitability were predicted following the complete loss of
vestibular function, recently Zheng et al. (2010) reported that this was not the case, at least in
vivo. Neither the baseline field potential, population spike height in the dentate gyrus, field
potential in the CA1 area, nor the induction or maintenance of long-term potentiation (LTP) in
vivo were affected by BVD in rats at 6 weeks or 7 months following surgery (Zheng et al.,
2010). The authors postulated that in spite of place cell dysfunction and interruption in theta
activity following BVD (Stackman et al., 2002; Russell et al., 2003b, 2006), the BVD-
induced spatial memory impairments may not be due to inability of the hippocampus to
generate LTP (Zheng et al., 2010).
Overall, these findings suggest that vestibular signals may exert an important influence
on spatial information processing in the hippocampus, which may explain, at least in part, the
navigational cognitive deficits of animals and humans with vestibular dysfunction. However,
knowledge of how this influence occurs is far from complete, and the findings outlined here
highlight a need for further research into the connection between the vestibular system and
hippocampal function.

Chapter 1: Introduction 19
1.2 The Endocannabinoid System
Marijuana (Cannabis sativa), or Cannabis, is one of the oldest drugs of abuse in the
world, but is also the drug with the longest recorded history of medicinal value. The major
psychoactive constituent of marijuana, 9-tetrahydrocannabinol (
9-THC), was identified by
Ganoi and Mechoulam (1964), who subsequently revealed its chemical structure and activity
(Mechoulam and Gaoni, 1967; Mechoulam, 1970; Mechoulam et al., 1970). Furthermore,
studies of the biological effects of 9-THC and its synthetic analogues have shown a precise
structural selectivity (Hollister, 1974) as well as stereo-selectivity (Jones et al., 1974),
foreshadowing the existence of a drug-receptor interaction. Besides marijuana‟s known
euphoric effect, its use also results in changes in learning and memory, anxiety, analgesia,
hypothermia, stimulation of food intake, anti-emetic effects, and vasorelaxation in humans
(see Jones, 1977; Maykut, 1985; Taylor, 1998; Hall and Degenhardt, 2009 for reviews). In
rodents, Cannabis and its related compounds have been shown to produce a characteristic
combination of up to four of the following symptoms: hypothermia, analgesia, hypoactivity
and catalepsy (Grunfeld and Edery, 1969; Compton et al., 1993; see Adams and Martin, 1996;
Chaperon and Thiébot, 1999 for review).
The discovery of 9-THC and the chemical synthesis of its analogues provided the
foundation for Cannabis research, and since then a growing body of evidence has emerged
focusing on the role of the endocannabinoid system in numerous diseases and disorders. The
endocannabinoid system is comprised of: 1) cannabinoid receptors, 2) endogenous
cannabinoids, also known as endocannabinoids, and 3) enzymes responsible for the
production, transport, and degradation of these cannabinoids and their receptors (see Elphick
and Egertova, 2001 for review).
1.2.1 Cannabinoid Receptors and Endocannabinoids
CB1 receptors
The first report that 9-THC might exert its effects by interacting with a specific
receptor protein in the brain was by Howlett (1984), when it was demonstrated that
cannabinoids decrease cyclic adenosine monophosphate (cAMP) levels in neuroblastoma cell
cultures, suggesting a Gi/o-coupled receptor-mediated action (Howlett and Fleming, 1984;
Howlett, 1985; Howlett et al., 1986). Further characterisation of this putative cannabinoid
receptor was carried out by Devane et al. (1988), who demonstrated the high-affinity,

Chapter 1: Introduction 20
saturable, stereospecific binding of a synthetic cannabinoid agonist, [1,2-(R)-5]-5-(1,1-
dimethyleptyl)-2-[5-hydroxy-2-(3-hydroxy propyl) cyclohexyl] phenol (CP-55,940) in rat
brain homogenates. Two years later, Herkenham and others performed an autoradiographic
study using tritiated CP-55,940 ([3H]-CP-55,940) to show the distribution of cannabinoid
binding sites in the brain (Herkenham et al., 1990, 1991). Following this, Matsuda and others
reported the cloning of a complimentary deoxyribonucleic acid (cDNA) sequence, encoding a
cannabinoid 1 receptor (CB1) from a rat brain cDNA library (Matsuda et al., 1990). Two
splice variants of the CB1 receptor have also been discovered: CB1A, which has an altered
amino-terminal sequence (Shire et al., 1995), and CB1B, which has an in-frame deletion of 33
amino acids at the amino-terminus (Ryberg et al., 2005).
The CB1 receptor is a seven transmembrane-domain receptor, coupled to the pertussis
toxin-sensitive G protein, Gi/o. As a member of the G protein-coupled receptor (GPCR)
superfamily, the actions of CB1 receptors are transduced via the activation of these G proteins
(Prather et al., 2000). CB1 receptor activation of G-proteins results in the inhibition of
adenylyl cyclase (Howlett, 1984), inhibition of calcium (Ca2+
) conductances (Mackie and
Hille, 1992; Caulfield and Brown, 1993; Mackie et al., 1995), stimulation of potassium (K+)
conductances (Deadwyler et al., 1995; Mackie et al., 1995), and stimulation of the mitogen-
activated protein kinase pathway (Bouaboula et al., 1995; see Demuth and Molleman, 2006
for review). The CB1 receptors are among the most abundant GPCRs in the rat and human
CNS (Herkenham et al., 1991; Mailleux and Vanderhaeghen, 1992; Glass et al., 1997), and
the density of CB1 receptors in the brain rivals that of ionotropic receptors for glutamate and
-aminobutyric acid (GABA) (see Herkenham, 1995 for review). Among the various brain
regions, the CB1 receptors are highly expressed in the basal ganglia, the molecular layer of the
cerebellum, the hippocampus, the cerebral cortex, the rostral ventromedial medulla, certain
nuclei of the thalamus and amygdala, the periaqueductal grey and dorsal primary afferent
spinal cord regions (Herkenham et al., 1990, 1991; Tsou et al., 1998; Ashton et al., 2004a;
Suarez et al., 2008). In the hippocampus, CB1 receptors have been found to be mainly
expressed in GABAergic inhibitory interneurons, most of which originate from
cholecystokinin-positive basket cells (Katona et al., 1999; Tsou et al., 1999; Egertova and
Elphick, 2000; Nyiri et al., 2005; Neu et al., 2007). Recently, the presence of presynaptic CB1
receptors has also been reported in hippocampal glutamatergic axon terminals, suggesting
their role in the inhibition of glutamate release (Katona et al., 2006; Kawamura et al., 2006;
Takahashi and Castillo, 2006). However, it is important to note that the expression of CB1
receptors in these excitatory synapses is at least 20 times lower than that at inhibitory

Chapter 1: Introduction 21
interneuron sites (Kawamura et al., 2006). CB1 receptors are also present, although at much
lower levels, in a variety of peripheral organs and tissues, among them being immune cells,
the spleen, the adrenal and pituitary glands, sympathetic ganglia, the heart, the lungs, and in
parts of the reproductive, urinary and gastrointestinal tracts (Galiègue et al., 1995; see
Pertwee, 1997 for review).
Following the initial anatomical evidence for the localisation of CB1 receptors in the
brain (Herkenham et al., 1990, 1991), subsequent light microscopy studies have revealed the
expression of numerous CB1 receptor-positive fibres throughout the brain (Egertova et al.,
1998; Tsou et al., 1998; Egertova and Elphick, 2000; Hájos et al., 2000; Katona et al., 1999,
2000, 2001). These fibres were tentatively identified as axons based on their distinctive
morphology (thin and rich in varicosities). This identification of presynaptic localisation was
then confirmed from work conducted in the rat hippocampus using electron microscopy,
where silver-impregnated gold particles represented the CB1-immunopositive sites (Katona et
al., 1999). The varicosities observed using light microscopy corresponded to axon terminals,
which were found to be densely covered with CB1 receptors and packed with synaptic
vesicles (Katona et al., 1999, 2000).
It has been shown that activation of the CB1 receptor causes the inhibition of the release
of the associated neurotransmitter, which may vary depending on the location of the CB1
receptor. One of the first studies of a cannabinoid receptor causing inhibition of
neurotransmitter release was carried out by Gill et al. in 1970. In this study, an electrically-
evoked twitch response of the guinea-pig ileum (which involves the release of acetylcholine)
was depressed by the application of 9-THC (Gill et al., 1970). Further evidence that the
activation of presynaptic CB1 receptors decreases the release of various neurotransmitters has
been demonstrated in in vitro assays as well as in various in vivo experimental animal models
and in humans (Gifford and Ashby, 1996; Gessa et al., 1997; Kathmann et al., 1999; Katona
et al., 1999; Gifford et al., 2000; Hájos et al., 200l; Szabo et al., 2002; Wang, 2003; Foldy et
al., 2006; Kawamura et al., 2006; Lee et al., 2010; see Schlicker and Kathmann, 2001 for
review).
CB2 receptors
Following the identification of the CB1 receptor, a second cannabinoid receptor subtype,
CB2, was isolated and cloned from the human promyelocytic cell line HL60 (Munro et al.,
1993). The CB1 and CB2 receptors share 44% overall identity in their primary protein

Chapter 1: Introduction 22
structure, showing the highest degree of homology in the transmembrane domains (see
Childers and Breivogel, 1998 for review). In contrast to presynaptic localisation of CB1
receptors in the brain, immunohistochemical studies suggest postsynaptic localisation of CB2
receptors (Gong et al., 2006; Onaivi et al., 2006), which has recently been confirmed by the
use of electron microscopy (Brusco et al., 2008b).
CB2 receptors are mainly expressed in cells of the immune and haematopoietic systems,
among them leukocytes, and cells in the spleen and the tonsils (Munro et al., 1993). Due to
the expression of CB2 receptors in the immune system, CB2 receptor activation elicits
immunomodulatory responses, such as a decrease in antigen presenting cell activity and a
down regulation of cytokine (IFN- and TNF-) production during inflammatory responses
(Klein et al., 2001; Lombard et al., 2007). However, there have also been recent
investigations showing that CB2 receptors are found in the CNS in response to injury. The
presence of CB2 receptors has been reported in glial cells associated with neuritic plaques in
Alzheimer‟s disease (Benito et al., 2003; Ramirez et al., 2005) and Down‟s syndrome brains
(Núñez et al., 2008), in the CNS of animals with experimental autoimmune encephalomyelitis
(an animal model of human multiple sclerosis) (Maresz et al., 2005), in plaque cell subtypes
in human multiple sclerosis (Benito et al., 2007), in brain tumours (Ellert-Miklaszewska et al.,
2007), in macrophages following middle cerebral artery occlusion in rats (Ashton et al., 2007),
and in activated microglia in an animal model of Parkinson‟s disease (Price et al., 2009).
From these results, it seems possible that CB2 receptor expression may be induced in discrete
brain regions following neuropathological insults. Furthermore, the expression of CB2
receptors has also been found in the brains of healthy subjects (Van Sickle et al., 2005; Ashton
et al., 2006; Gong et al., 2006; Onaivi, et al., 2006; Baek et al., 2008). Consequently, the
original concept that CB1 receptors play an exclusive role in the brain, and CB2 receptors in
the immune system, has evolved into the idea that both cannabinoid receptor types can be
involved in controlling both central and peripheral functions. Moreover, functional studies
have suggested that the activation of CB2 receptors by the administration of exogenous CB2
receptor ligands may open novel therapeutic avenues for a number of pathological CNS
conditions such as Alzheimer‟s disease, stroke, and neuropathic pain (Ramirez et al., 2005;
Zhang et al., 2007, 2008; Jhaveri et al., 2008; see Rivers and Ashton, 2010 for review).
Endocannabinoids
The discovery of the cannabinoid receptors opened the way for the identification of
their endogenous ligands, known as „endocannabinoids‟. Endocannabinoids represent a class

Chapter 1: Introduction 23
of lipophilic compounds based on the general structure of modified arachidonic acid
derivatives. Arachidonyl ethanolamide or anandamide (AEA) and 2-arachidonyl glycerol (2-
AG) are the two best characterised endocannabinoids in the mammalian CNS (see Piomelli,
2003 for review). AEA was isolated in 1992 by Devane et al., and it acts as a partial agonist at
both CB1 and CB2 receptors (Sugiura et al., 2002). The other major endocannabinoid, 2-AG,
was initially isolated from the canine gut (Mechoulam et al., 1995) and the rat brain (Sugiura
et al., 1995). The concentration of 2-AG in the mammalian brain is much higher than that of
AEA, and it activates both CB1 and CB2 receptors with greater efficacy than does AEA (see
Sugiura et al., 2006; Sugiura, 2009 for reviews). Other endocannabinoids have also been
isolated during the last decade, including 2-arachidonyl-glycerol ether (noladin ether) (Hanus
et al., 2001), N-arachidonoyl-dopamine (NADA) (Bisogno et al., 2000; Huang et al., 2002)
and virodhamine (Porter et al., 2002). However, the pharmacological and physiological
activities of these endocannabinoids have not yet been fully characterised.
Due to the lipophilic nature of AEA and 2-AG, they are not stored in the aqueous
interior of synaptic vesicles, but are synthesised and released from postsynaptic neurons on
demand, and target presynaptic cannabinoid receptors (Di Marzo et al., 1994; Stella et al.,
1997; see Kano et al., 2009 for review; Figure 1.6). There have been two major pathways
described for the activation of endocannabinoid synthesis from postsynaptic neurons. These
are the phospholipase C (PLC)-dependent pathway and the Ca2+
-dependent pathway (see
Heifets and Castillo, 2009 for review; Figure 1.6). The PLC-dependent pathway is triggered
by the activation of Gq-coupled receptors including group 1 metabotropic glutamate receptors
(mGluRs) (Maejima et al., 2001; Varma et al., 2001; Ohno-Shosaku et al., 2002) and M1/M3
muscarinic receptors (Kim et al., 2002; Ohno-Shosaku et al., 2003; Fukudome et al., 2004).
The other described pathway, the Ca2+
-dependent pathway, is driven by an increase in
intracellular Ca2+
concentration via activation of voltage-gated Ca2+
channels (Brenowitz and
Regehr, 2003; Adermark and Lovinger, 2007). AEA is synthesised from a phospholipid
precursor, N-arachidonoyl-phosphtidyl ethanolamine (NArPE), which is catalysed by N-acyl
phosphatidyl ethanolamine phospholipase D (NAPE-PLD; Figure 1.6). The synthesis of 2-AG
occurs through the formation from phospholipids of a diacylglycerol (DAG) precursor, which
is catalysed by PLC, followed by the hydrolysis of DAG lipase (DAGL; Figure 1.6).

Chapter 1: Introduction 24
Figure 1.6
Schematic diagram of the synthesis, signalling, and degradation of endocannabinoids AEA and
2-AG at the synaptic cleft. Abbreviations are: AEA, anandamide; 2-AG, 2-arachidonoyl glycerol;
DAGL, diacylglycerol lipase; EC, endocannabinoid; EMT, endocannabinoid membrane
transporter; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase; mGluR,
metabotropic glutamate receptor; M1/M3, muscarinic receptor types M1 and M3; NAPE-PLD, N-
acyl phosphatidyl ethanolamine phospholipase D; NArPE, N-arachidonoyl-phosphtidyl
ethanolamine; PLC, phopholipase C. Figure reproduced from Lutz and Marsicano (2009),
with copyright permission from Elsevier.
Endocannabinoids are removed from synaptic clefts by a two-step mechanism: re-
uptake and subsequent intracellular enzymatic degradation (Hillard and Jarrahian, 2000;
Fowler and Jacobsson, 2002; McFarland and Barker, 2004). There are at least three models
proposed for AEA uptake by cells. The first model is that AEA is transported via the
endocannabinoid membrane transporter (EMT), which transports AEA from one side of the
membrane to the other (Fegley et al., 2004; Ligresti et al., 2004). The second model is that
AEA passes through the membrane by simple diffusion, which is facilitated by the

Chapter 1: Introduction 25
concentration gradient resulting from intracellular degradation (Glaser et al., 2003). The third
model is that AEA undergoes endocytosis through a caveolae-related uptake process
(McFarland et al., 2004). Contrary to extensive investigation of the AEA re-uptake
mechanism, there is relatively little known about 2-AG uptake. However, there are several
studies suggesting that the 2-AG uptake mechanism is similar to that of AEA (Piomelli et al.,
1999; Beltramo and Piomelli, 2000; Bisogno et al., 2001). Once the endocannabinoids are
taken into a cell, AEA is catalysed by fatty acid amide hydrolase (FAAH) and 2-AG by
monoacylglycerol lipase (MAGL) in addition to FAAH (Goparaju et al., 1998; Deutsch et al.,
2001; Glaser et al., 2003; Day et al., 2001; Figure 1.6).
Overall, the postsynaptic release of endocannabinoids, together with the presence of
presynaptic CB1 receptors on both the inhibitory and excitatory synapses, suggests that
endocannabinoids play an important role in modulating synaptic transmission in the nervous
system.
1.2.2 Neuromodulatory Role of the Endocannabinoid System
The role of endocannabinoids as retrograde messengers mediating short-term (< 1
minute) inhibition of neurotransmitter release was first postulated in studies by Llano et al.
(1991) and Alger (Pitler and Alger, 1992). In these studies, a brief depolarisation of principal
neurons (Purkinje cells of the cerebellum and pyramidal cells of the hippocampus) triggered a
transient suppression of GABAergic synaptic input in the cerebellum (Llano et al., 1991) and
in the hippocampus (Pitler and Alger, 1992). This phenomenon was termed depolarisation-
induced suppression of inhibition (DSI). Over the next decade, numerous studies were
published confirming that endocannabinoids were the “mysterious” retrograde messengers
that mediated cerebellar (Kreitzer and Regehr, 2001a; Diana et al., 2002; Yoshida et al., 2002)
and hippocampal (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001) DSI. Furthermore,
Kreitzer and Regehr (2001b) discovered an analogous phenomenon, termed depolarisation-
induced suppression of excitation (DSE) in the cerebellum, which was also shown to be
mediated by endocannabinoids acting upon glutamatergic synapses. More recently, DSE has
also been demonstrated in the dentate gyrus region of the hippocampus (Chiu and Castillo,
2008). Taken together, these results demonstrate that endocannabinoids are important
mediators of short-term plasticity in the brain.
It has been shown that endocannabinoids have the capacity for inducing not only short-

Chapter 1: Introduction 26
term, but also long-term synaptic plasticity (minutes to hours) in the brain, which has
indicated the potential long-term effect of endocannabinoids on brain function (see Gerdeman
and Lovinger, 2003; Chevaleyre et al., 2006 for reviews). Endocannabinoid-mediated long-
term plasticity takes the form of depression, in which the activation of presynaptic CB1
receptors by endocannabinoids causes a long-lasting reduction of neurotransmitter release.
This endocannabinoid-mediated long-term depression (LTD) has been shown to be a
widespread phenomenon in the brain, that has been observed at both excitatory (glutamatergic)
(Gerdeman et al., 2002; Robbe et al., 2002; Sjostrom et al., 2003; Mato et al., 2008; Haj-
Dahmane and Shen, 2010) and inhibitory (GABAergic) (Chevaleyre and Castillo, 2003;
Chevaleyre et al., 2007; Adermark et al., 2009) synapses in various brain structures including
the dorsal striatum (Gerdeman et al., 2002; Adermark et al., 2009), the nucleus accumbens
(Robbe et al., 2002; Mato et al., 2008), the amygdala (Chevaleyre et al., 2007), the
hippocampus (Chevaleyre and Castillo, 2003; Chevaleyre et al., 2007; see Lafourcade, 2009
for review), the neocortex (Sjostrom et al., 2003), and the ventral tegmental area (Szabo et al.,
2002; Haj-Dahmane and Shen, 2010; see Heifets and Castillo, 2009 for review). Interestingly,
persistent CB1 receptor activation is not required to maintain endocannabinoid-mediated LTD.
This phenomenon was demonstrated in studies which showed that, once established,
endocannabinoid-mediated LTD was not reversed by the administration of CB1 receptor
inverse agonists, such as N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-
methyl-1H-pyrazole-3-carboximide hydrochloride (SR141716A) and N-(piperidin-1-yl)-5-(4-
iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboximide hydrochloride
(AM251) (Robbe et al., 2002; Sjostrom et al., 2003; Chevaleyre and Castillo, 2003; Ronesi et
al., 2004; Adermark et al., 2009).
Reflecting the widespread distribution of CB1 receptors in the brain (Herkenham et al.,
1990; Tsou et al., 1998), administration of exogenous agonists for the CB1 receptors also
reliably suppresses synaptic transmission in various regions of the brain. These regions
include the hippocampus (Shen et al., 1996; Hoffman and Lupica, 2000; Chiu and Castillo,
2008; Bajo et al., 2009; Serpa et al., 2009), the substantia nigra pars compacta (Chan et al.,
1998; Szabo et al., 2000; Wallmichrath and Szabo, 2002), the cerebellum (Levenes et al.,
1998; Daniel et al., 2004; Kawamura et al., 2006), and the nucleus accumbens (Pistis et al.,
2002; Robbe et al., 2003). This suppression of synaptic transmission by exogenous CB1
receptor agonists has been shown to occur at both excitatory (Kawamura et al., 2006; Bajo et
al., 2009; Fan et al., 2010) and inhibitory (Foldy et al., 2006; Inada et al., 2010; Lee et al.,
2010) synapses. Administration of CB1 receptor inverse agonists prevented or reversed

Chapter 1: Introduction 27
cannabinoid-induced suppression of synaptic transmission, which confirmed that exogenous
cannabinoids act via the CB1 receptors (Wallmichrath and Szabo, 2002; Bajo et al., 2009;
Serpa et al., 2009; Inada et al., 2010; Lee et al., 2010). Furthermore, administration of
cannabinoid receptor agonists in CB1 receptor knock-out mice has been observed not to
produce any suppression of synaptic transmission, which is evidence further demonstrating
the involvement of CB1 receptors in the modulation of synaptic transmission (Kawamura et
al., 2006; Fan et al., 2010). Aside from synaptic changes, CB1 receptor activation has also
been shown to modulate synaptic plasticity by influencing neurogenesis in the hippocampus,
as the activation of CB1 receptors has been found to promote neurogenesis in the
hippocampus in animals (Jin et al., 2004; Marchalant et al., 2009; Wolf et al., 2010).
Overall, cannabinoid-mediated synaptic plasticity via CB1 receptors is a widespread
phenomenon in the brain, and it reflects the role of the endocannabinoid system in brain
function.
1.2.3 The Role of the Endocannabinoid System in Cognitive Function
In the years since the identification of cannabinoid receptors and the development of
synthetic cannabinoids, there has been significant progress in assessing the effects of
cannabinoids on cognitive processes. Impairments in learning and memory are among the best
known behavioural effects of cannabinoids. In humans, it has long been recognised that
thoughts become fragmented and short term memory is impaired following Cannabis intake
(Bromberg, 1934; Ames, 1958; Abel, 1971; Miller and Branconnier, 1983; Heishman et al.,
1997; Nestor et al., 2008; Weinstein et al., 2008; see Ranganathan and D'Souza, 2006 for
review). Similarly, the disruptive effects of cannabinoid receptor agonists on learning and/or
on the performance of diverse memory tasks have also been thoroughly documented in non-
human primates (Zimmerberg et al., 1971; Aigner, 1988; Winsauer et al., 1999; Nakamura-
Palacios et al., 2000) and in rodents (Carlini et al., 1970; Litchman et al., 1995; Ferrari et al.,
1999; Hampson and Deadwyler, 2000; Cha et al., 2006; Barna et al., 2007; Pamplona et al.,
2008; Suenaga and Ichitani, 2008).
The hippocampus is a brain structure that has been strongly implicated in learning and
memory in both animals and in humans (Altemus and Almli, 1997; Whishaw et al., 2001;
Brandt et al., 2005; Iordanova et al., 2009; Goodrich-Hunsaker et al., 2010). There is
evidence to suggest that the effects of cannabinoids on learning and memory may be due, at

Chapter 1: Introduction 28
least in part, to effects of cannabinoids on the hippocampus. Not only are CB1 receptors
highly expressed in the hippocampus (Herkenham et al., 1990; see Mackie, 2005 for review),
but also a great deal of evidence demonstrates that the activation of these receptors results in
impairment in various memory tasks that are known to be sensitive to disruption of
hippocampal functions in animals. For example, systemic or intrahippocampal administration
of several CB1 receptor agonists led to impaired performance by rats in the Morris water maze
(Robinson et al., 2003, 2007; Yim et al., 2008), the radial arm maze (Lichtman et al., 1995;
Egashira et al., 2002; Suenaga et al., 2008; Wegener et al., 2008; Wise et al., 2009), and an
object recognition task (Schneider and Koch, 2002; Barna et al., 2007; Clarke et al., 2008;
Schneider et al., 2008; Suenaga and Ichitani, 2008; Baek et al., 2009). The memory-impairing
effects of CB1 receptor agonists have been shown to be mediated at least in part via the CB1
receptors, as these agonist-induced deficits are reversed by the administration of CB1 receptor
inverse agonists such as SR141716A and AM251 (Lichtman and Martin, 1996; Brodkin and
Moerschbaechier, 1997; Chaperon et al., 1998; Mallet and Beninger, 1998; Da Silva and
Takahashi, 2002; Suenaga et al., 2008; Wise et al., 2009). Furthermore, these cannabinoid-
induced memory impairments resemble the effects of hippocampal lesions on learning and
memory in humans (Kessels et al., 2001; Spiers et al., 2001; Astur et al., 2002; Goodrich-
Hunsaker et al., 2010) and in rats (Altemus and Almli, 1997; Gilbert et al., 2002; Broadbent
et al., 2004; Talpos et al., 2008; Faraji et al., 2008; Iordanova et al., 2009; Brady et al., 2010).
For these reasons, the detrimental effects of CB1 receptor agonists on learning and memory
have been attributed primarily to their effect on hippocampal function.
As treatment with CB1 receptor agonists produces memory impairment, it has been
hypothesised that blocking CB1 receptors might enhance or improve memory. However, the
effects of CB1 receptor inverse agonists in rats have been inconsistent. While in some studies
the CB1 receptor inverse agonists such as SR141716A and AM251, seemed to enhance
memory (Terranova et al., 1996; Takahashi et al., 2005; Lichtman, 2000; Wolff and Leander,
2003; de Oliveira Alvares et al., 2008), other studies have shown no effect (Brodkin and
Moerschbaecher, 1997; Da and Takahashi, 2002; Suenaga et al., 2008) or even impairment of
memory (de Oliveira Alvares et al., 2005, 2006; Arenos et al., 2006). Furthermore, studies
with mice lacking CB1 receptors showed that these animals have a prolonged aversive
memory and suggested that the endocannabinoid system may have a role in facilitating
extinction and/or forgetting processes (Reibaud et al., 1999; Varvel and Lichtman, 2002;
Marsicano et al., 2002; Varvel et al., 2005; see Valverde et al., 2005 for review).

Chapter 1: Introduction 29
Modifications of the strength of synaptic connections between neurons have been
shown to underlie the mechanism of memory formation and storage in the mammalian brain
(see Maren and Baudry, 1995 for review). LTP and LTD are forms of synaptic plasticity in the
hippocampus which have been suggested to have a role in learning and memory processes
(see Martin et al., 2000 for review). Interestingly, previous studies have shown that the
activation of CB1 receptors in hippocampal slices, either by synthetic cannabinoids or by
endocannabinoids, impairs LTP (Collins et al., 1994, 1995; Terranova et al., 1995; Stella et al.,
1997; Lees and Dougalis, 2004; Hoffman et al., 2007; Bajo et al., 2009) and LTD (Misner and
Sullivan, 1999; Mato et al., 2004; see Chevaleyre et al., 2006 for review), indicating the
involvement of the hippocampal CB1 receptors in learning and memory processes.
Furthermore, in vivo studies have demonstrated the disruption of hippocampal neuronal
function and responsiveness following the administration of CB1 receptor agonists (Hampson
and Deadwyler, 2000; Robbe et al., 2006; Robinson et al., 2007; Abush and Akirav, 2009;
Robbe and Buzsaki, 2009; Goonawardena et al., 2010). For example, hippocampal
multineuron recordings revealed that the administration of 9-THC or (R)-(+)-[2,3-dihydro-5-
methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin -6-yl]-1-
naphthalenylmethanone (WIN 55212-2; a synthetic cannabinoid CB1/CB2 receptor agonist)
dose-dependently reduced the firing rate of hippocampal principal cells during the encoding
(sample) phase of a delayed nonmatch to sample (DNMS) task (Hampson and Deadwyler,
2000). This result suggests that the encoding of information by hippocampal neurons is
important in the performance of the DNMS task (Hampson and Deadwyler, 2000). Similarly,
Robinson and others showed that the administration of the synthetic cannabinoid agonist
(6aR,10aR)-9-(Hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10,a-
tetrahydrobenzo [c]chromen-1-ol (HU-210), resulted in reductions in firing frequency, and in
the bursting of CA1 and CA3 principal neurons of the hippocampus, which were proposed as
underlying mechanisms for the spatial memory deficits observed with the same drug
(Robinson et al., 2007). Overall, these results show that the endocannabinoid system plays an
important role in cognitive function.

Chapter 1: Introduction 30
1.3 The Association Between the Vestibular and Endocannabinoid
Systems
As previously explained (see sections 1.1.4 Vestibular-Hippocampal Connection and
1.2.3 The Role of the Endocannabinoid System in Cognitive Function), it has been suggested
that both the vestibular and endocannabinoid systems play a significant role in cognitive
function in animals and humans. Furthermore, numerous human (Scoville and Milner, 1957;
Frisk and Milner, 1990; Burgess et al., 2002; Maguire et al., 2006; Goodrich-Hunsaker et al.,
2010) and animal (Olton and Werz, 1978; Altemus and Almli, 1997; Forwood et al., 2005;
Talpos et al., 2008; Faraji et al., 2008; Iordanova et al., 2009) studies demonstrate that
hippocampal integrity is important for normal learning and memory functions which require
ongoing neuroplastic modifications, such as the formation of new synapses or a
reorganization of existing synpases (Bailey and Kandel, 1993; Moser, 1999; Rubino et al.,
2009b). Interestingly, cognitive deficits and alterations in hippocampal synaptic plasticity
following either cannabinoid receptor agonist treatment or BVD show similarities in both
experimental animal models and in human patients. A number of behavioural studies have
shown that both cannabinoid receptor agonist administration (Lichtman et al., 1995; Egashira
et al., 2002; Robinson et al., 2007; Wegener et al., 2008; Wise et al., 2009) and BVD
(Wallace et al., 2002c; Russell et al., 2003a; Schautzer et al., 2003; Brandt et al., 2005; Zheng
et al., 2007, 2009b) cause spatial memory impairment in hippocampus-dependent tasks in
both animals and humans. Such results suggest that the spatial memory deficits may, at least
in part, be due to detrimental effects of cannabinoids and BVD on the hippocampus.
Furthermore, as it has been demonstrated that many hippocampal synaptic changes are seen in
the brain after vestibular damage (Stackman et al., 2002; Russell et al., 2003b, 2006; Zheng et
al., 2010) and that the endocannabinoid system is critically involved in synaptic plasticity in
the hippocampus (see Mackie, 2008 for review), it is possible that the endocannabinoid
system may be contributing significantly to the hippocampal plasticity observed following
lesions of the vestibular system.

Chapter 1: Introduction 31
1.4 Research Goals
Although extensive research has been carried out on the vestibular system, the focus in
the past has been largely on the role of the vestibular system in the ocular motor and postural
reflexes, as these reflexive movements are directly associated with vestibular function
(Honrubia et al, 1984, 1985; Zennou-Azogui et al., 1993; Hamann et al., 1998; Mbongo et al.,
2009). However, there has been a persistent accumulation of evidence showing detrimental
effects of vestibular damage on cognitive functions, including attention and, learning and
memory in both animals and humans (Wallace et al., 2002c; Russell et al., 2003a; Brandt et
al., 2005; Zheng et al., 2009a, b). This has led to more quantitative investigations, and
eventually to studies of the effects of peripheral vestibular damage on the hippocampus.
While both the vestibular and endocannabinoid systems have been investigated in great
detail, no previous study has investigated the possible involvement of the endocannabinoid
system in the cognitive impairments that result from vestibular dysfunction. Therefore, the
aim of this research was to determine whether the hippocampal endocannabinoid system plays
a role in the functional changes seen after vestibular damage. By better understanding the
mechanism(s) of this phenomenon, it may be possible to minimise this pathological process,
and also to develop potential treatments which can restore cognitive function.
Therefore, the goals of this research were to:
1) Determine the optimum memory-impairing dose of the cannabinoid CB1/CB2 receptor
agonist, WIN 55212-2 („WIN‟), in an object recognition task, prior to investigating its
effects on the cognitive deficits in BVD rats (Chapter 2),
2) Determine whether BVD-induced spatial deficits would persist at 14 months following
BVD and whether any spatial deficits apparent at 14 months post-BVD could be
modulated by the administration of WIN, or the CB1 receptor inverse agonist, AM251
(Chapter 3),
3) Determine whether the density and the affinity of CB1 receptors in the hippocampus
changes following BVD (Chapter 4).

32
Chapter 2
The Effect of the Cannabinoid Receptor Agonist, WIN
55212-2, on Object Recognition Memory in Rats

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 33
2.1 Introduction
It was necessary to determine the optimum dose of the cannabinoid CB1/CB2 receptor
agonist, WIN 55212-2 (WIN), prior to investigating its effect on the cognitive deficits in rats
induced by bilateral vestibular deafferentation (BVD). The object recognition memory task
was chosen to achieve this goal as this particular behavioural paradigm does not require any
pre-training, and therefore the effects of WIN in rats could be determined in a relatively short
period of time.
Learning and memory impairments are among the most renowned effects of
cannabinoids in both humans and animals (see Adams and Martin, 1996; Ameri, 1999; Diana
and Marty, 2004 for reviews). In humans, the cannabinoid receptor agonist 9-THC produces
a variety of “intoxicating” psychoactive and motor effects, including a marked impairment
and disruption of short-term memory as well as several other perceptual and disorientation
effects (Miller and Branconnier, 1983; Murray, 1986; Chait and Perry, 1992). In addition,
chronic use of marijuana also leads to a lasting impairment in cognition (Solowij et al., 1995;
Pope and Yurgelun-Todd, 1996; Pope et al., 2001), which may be due to changes in
hippocampal morphology (Lawston et al., 2000; Tagliaferro et al., 2006). Numerous
behavioural studies in animals have shown that systemic or intrahippocampal administration
of natural cannabinoid receptor agonists like 9-THC or anandamide, or synthetic agonists,
such as WIN, CP-55,940 or HU-210, impairs performance in hippocampus-dependent
memory tasks, such as the radial arm maze (Nakamura et al., 1991; Lichtman et al., 1995;
Suenaga et al., 2008; Wise et al., 2009), the water maze (Robinson et al., 2003; Marchalant et
al., 2008), and the delayed alternation task (Heyser et al., 1993;Nava et al., 2000, 2001;
Suenaga et al., 2008).
Recognition memory, although it can be defined in many ways, is generally regarded as
the ability to discriminate the familiarity of items or events that have previously been
encountered and is considered to be a critical component of declarative memory (Mumby,
2001). Declarative memory is characterised as the conscious memory for facts and events and
is further divided into episodic memory (memory for personal events) and semantic memory
(memory for general information; Squire and Zola, 1996; Winters et al., 2008). On the other
hand, procedural memory for habits or skills is defined as a non-declarative memory, and
requires an extensive acquisition phase (Winters et al., 2008). Declarative memory can be
acquired with relatively fewer exposures to the material to be learned, compared to procedural

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 34
memory.
Object recognition memory is commonly impaired in amnesic patients who lose their
ability to recognise people and objects they have come across since the onset of amnesia. For
the past 20 years, a variety of behavioural methods for studying object-recognition memory in
animals have been developed, which include the delayed nonmatching-to-sample (DNMS)
task, delayed matching-to-sample (DMS) task, and spontaneous object recognition (SOR) task
(see Mumby, 2001; Winters et al., 2008 for reviews).
The SOR task is a variation of the DNMS paradigm to assess object recognition
memory. It is referred to as spontaneous because it exploits the innate tendency of rats to
explore novel stimuli in preference to familiar stimuli (Ennaceur and Delacour, 1988).
Rodents readily approach objects and investigate them physically by touching and sniffing the
objects, rearing upon and trying to manipulate them with their forepaws (Aggleton, 1985).
The similarity between the DNMS and SOR tasks is that the behaviour of normal animals in
the test or choice phase is driven by a single exposure to sample objects and the subsequent
recognition (Winters et al., 2008). In the standard version of the SOR task, a rat is placed in
an open field arena and allowed to explore the sample objects in the arena for a fixed duration,
usually few minutes (5-10 minutes). This period is called the sample phase. The rat is then
taken away from the arena for a retention delay, which lasts several minutes to hours, and then
returned to the arena with one of the sample objects changed to a novel one. This is called the
test phase. By nature, rats should spend more time exploring the novel object than previously
encountered objects (Dere et al., 2007). Therefore, when there is a bias in time towards
exploring the novel objects compared to sample objects, it is suggested that the rat remembers
the sample objects and therefore this can be taken as an index of recognition of the familiar
sample object. The major advantage of this task over food-rewarding maze learning tasks and
classical DNMS or DMS tasks, is that it does not require spatial learning, food or water
deprivation, the application of any explicit reinforcement (food or electric shock delivery), the
learning of test rules or of response-reward correlations. Therefore, the study can be done
with little or no training and it is also less stressful for the animals than the negative
reinforcement-based tasks, such as the hidden platform version of the water maze, inhibitory
and active avoidance, or fear conditioning tasks, which have been extensively utilised to study
the neurobiology of learning and memory in rodents (see Winters et al., 2008 for review).
Many experimental paradigms, such as Morris water maze, radial arm maze, and

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 35
instrumental discrimination tasks, have been employed to investigate the role of cannabinoids
in learning and memory. However, the majority of these paradigms are based on conditioning,
yet recognition memory is a non-conditioned behaviour. Therefore, the evaluation of the
influence of cannabinoids on recognition memory can further provide evidence regarding the
involvement of the endocannabinoid system in learning and memory processes. Recognition
memory impairments induced by the systemic administration of cannabinoid receptor agonists
have been reported previously (Ciccocioppo et al., 2002; Schneider and Koch, 2002; Kosiorek
et al., 2003; Schneider et al., 2008). Furthermore, there are many studies involving direct
administration of cannabinoids into the hippocampus that have investigated performance in
the SOR task (Barna et al., 2007; Clarke et al., 2008; Suenaga and Ichitani, 2008). Although
some studies have reported object recognition deficits in adult rats (Schneider and Koch, 2002;
O‟Shea et al., 2006; Barna et al., 2007), most have reported that pubertal or pre-pubertal
exposure to CB1 receptor agonists is more detrimental (Schneider et al., 2005, 2007; O‟Shea
et al., 2006; Quinn et al., 2008). Furthermore, these studies support the notion that exposure
to Cannabis sativa during adolescence may predispose humans to cognitive impairment and
possibly even psychosis, later in life (Schneider and Koch, 2003; Schneider et al., 2008).
The aim of this study was to further investigate the issue of whether exposure to a CB1
receptor agonist affects rats during adulthood, by examining the dose-dependent effects of
WIN in an object recognition task in adult rats. Since most studies have employed multiple
injections (O‟Shea et al., 2004, 2006; Schneider and Koch, 2003, 2005, 2007; Quinn et al.,
2008; Schneider et al., 2008), the second issue of interest was whether a single injection of
the drug would interfere with object recognition. Furthermore, the results from the present
study would reveal the optimum dose of WIN for rats, to then be used to treat BVD animals
during performance in the foraging task (Chapter 3).

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 36
2.2 Materials and Methods
All experiments described throughout this thesis were approved by the University of
Otago Animal Ethics Commiittee (07/06). The animals were treated with respect and every
effort was made to ensure their wellbeing. In order to minimise the number of animals
involved in the study, efforts were made to maximise the data yield from each animal. The
animals were maintained on a twelve-hour light-dark cycle at 22o C and housed four per cage.
All experimental procedures throughout this thesis were conducted during the light period of
the light-dark cycle.
2.2.1 Animals
Twenty-nine naïve adult male Wistar rats (7 months old), weighing 500-600 g, were
used for this study. These animals were taken from another project in which the animals were
not exposed to any drug. Animals were randomly divided into four groups: (1) vehicle control
(n = 7); (2) 1 mg/kg WIN (n = 7); (3) 3 mg/kg WIN (n = 7); (4) 5 mg/kg WIN (n = 8).
2.2.2 Drugs
The cannabinoid agonist, (R)-(+)-[2,3-dihydro-5-methyl-3-(4-
morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin -6-yl]-1-naphthalenylmethanone (WIN
55212-2, WIN) was purchased from Tocris Bioscience (Bristol, UK). WIN was dissolved in
dimethyl sulfoxide (DMSO, Merck) and diluted in saline (0.9% sodium chloride), at a final
concentration of 1:1 ratio (DMSO:Saline). The drug was administered subcutaneously (s.c.) at
doses of 1, 3, or 5 mg/kg. The doses utilised were determined based on previous studies
showing the effects of cannabinoids on learning and memory (Lichtman et al., 1995;
Schneider, M. & Koch, M. 2002; Robinson et al., 2003; Chambers et al., 2004; Bhatti et al.,
2009; Shoaib, 2008). The vehicle solution was prepared in a similar manner, with the
exception that the drug was omitted (50% DMSO in saline). Each animal was weighed on the
day of injection and received a single dose of WIN or vehicle, 30 minutes prior to the testing
session.
2.2.3 Object Recognition Memory Test
The test was modified from Zheng et al. (2004). The duration of testing was three days.

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 37
The test was carried out in a 60 x 60 x 35 cm grey-painted wooden box, in which rats were
tested individually (Figure 2.1d). A video camera was mounted on the ceiling directly above
the testing apparatus to record animals‟ behaviour for future analysis. On day 1, each animal
was placed in the box for 10 minutes for habituation. No objects were placed in the arena
(Figure 2.1a). On day 2, each animal had two identical 10 minute sessions exploring four
identical plastic objects (4 x 6 cm) placed in the box (Figure 2.1b). The inter-trial interval was
30 minutes. The location of each object was kept constant for each rat and counter-balanced
across the rats and groups. All objects and the test arena were cleaned with diluted
disinfectant and thoroughly dried before each session and between each rat. On day 3, animals
were tested for their reaction to novelty. In the first session of day 3, animals were placed in
the arena with identical conditions to the previous day (Figure 2.1b). After the first session, a
subcutaneous injection of WIN or vehicle was given to each rat. Thirty minutes later, the
second session was run, in which one of the sample objects was replaced with a novel one
(Figure 2.1c). The location of the novel object was carefully randomised across the groups in
order to prevent location effects for particular groups. In another words, different groups
received equal exposure to the novel object in the different spatial position. The exploration
was defined as the duration of time the animal spent directing its nose at a distance within 2
cm of the object and/or touching the object. The total exploration time and time spent
exploring each object was recorded and analysed. Following Winters et al. (2004), a
discrimination index, defined as the difference between the time spent exploring the novel
versus the familiar objects, divided by the total time spent exploring objects, was also
calculated in order to control for the general effects of WIN on object exploration. A decrease
in the discrimination index would indicate a decline in an animal‟s ability to discriminate the
novel object from the familiar ones.
2.2.4 Statistical Analysis
Prior to any analysis, the data were tested for the parametric statistical assumptions of
normality and homogeneity of variance. The data were found to violate these assumptions and
therefore were square root transformed. Two-way analyses of variance (ANOVAs) with
repeated measures on time were then performed followed by Tukey‟s post-hoc comparisons
(Winer et al., 1991). The discrimination index data were subjected to a one-way ANOVA
followed by Tukey‟s post-hoc comparisons (Winer et al., 1991). P < 0.05 was considered
significant in all cases.

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 38
a) Day 1 b) Day 2 & 1st Session of Day 3
c) 2nd
Session of Day 3 d) Test Apparatus
Figure 2.1
The object recognition memory testing conditions for each day. (a) Habituation in the testing
arena without any objects on day 1. (b) Sampling phase with 4 identical objects (red circles) on
day 2 and on first session of day 3. (c) Reaction to novelty on day 3, where one of the sample
objects is changed to a novel one. (d) Photograph of object recognition memory task testing
arena with a novel and sample objects.

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 39
2.3 Results
2.3.1 Total Exploration Time
The total exploration time was significantly reduced with the administration of WIN in
a dose dependent manner (F(3,50) = 3.09, P = 0.035; Figure 2.2). When the novel object was
introduced in session 2, there was an apparent increase in the exploration time in the vehicle-
treated group. However, this increase was not significant as there was no significant session
effect or any significant interaction between the drug and the session. Post hoc comparisons
revealed that only 5 mg/kg WIN-treated animals spent significantly less time overall
exploring objects (both familiar and novel) compared to the vehicle treated animals (P =
0.033; Figure 2.2).
Figure 2.2
Total exploration time (in seconds) for session 1 (before drug administration) and session 2
(after drug administration) for the vehicle treated group and groups receiving 1, 3 or 5 mg/kg
WIN. Data are represented as means + SEM. There was a significant difference between the
vehicle and the 5 mg/kg WIN group only (* P = 0.033).
0
10
20
30
40
50
60
Vehicle 1 mg/kg WIN 3 mg/kg WIN 5 mg/kg WIN
Tota
l E
xp
lora
tion
Tim
e (
s)
Session 1
Session 2*

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 40
2.3.2 Exploration Time by Objects
When the exploration time towards each object was analysed, WIN-treated animals
spent significantly less time exploring the novel object compared to vehicle-treated animals
(F(3,50) = 7.06, P = 0.000; Figure 2.3). However, animals in all groups spent a longer time
exploring the novel object than the familiar object (F(1,50) = 53.55, P = 0.000) and there was a
significant interaction between the drug and the object (F(3,50) = 6.02, P = 0.001; Figure 2.3).
This suggests that the increase in the exploration time for the novel object was smaller for the
WIN-treated animals. Post hoc comparisons indicated that the time spent exploring the novel
object for the 5 mg/kg WIN-treated group was significantly lower than for the vehicle- (P =
0.0005) and 1 mg/kg WIN-treated groups (P = 0.008).
Figure 2.3
Mean exploration time (in seconds) for familiar and novel objects for the vehicle-treated group
and the groups receiving 1, 3 or 5 mg/kg WIN. Data are represented as means + SEM. Post hoc
comparisons indicated that the time spent exploring the novel object for the vehicle group was
significantly greater than for the 5 mg/kg WIN group (* P = 0.0005), and exploration time of the
novel object for the 1 mg/kg WIN group was significantly greater than for the 5 mg/kg WIN
group (* P = 0.008).
0
10
20
30
40
50
Vehicle 1 mg/kg WIN 3 mg/kg WIN 5 mg/kg WIN
Exp
lora
tion
Tim
e b
y O
bje
cts
(s) Novel
Familiar
*
*

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 41
2.3.3 Discrimination Index
The discrimination index was calculated and analysed in order to control for the general
effect of WIN on object exploration. Vehicle-treated animals showed a significantly higher
discrimination index compared to the three WIN groups (F(3,28) = 3.51, P = 0.03; Figure 2.4).
Post hoc comparisons showed that this was due to the 1 mg/kg WIN-treated group having a
significantly lower discrimination index than the vehicle group (P = 0.028).
Figure 2.4
The discrimination indices for the vehicle-treated group and the groups receiving 1, 3 or 5
mg/kg WIN. Data are represented as means + SEM. The discrimination index was significantly
lower in the 1 mg/kg WIN-treated group compared to the vehicle group (* P = 0.028), while there
were no differences between the discrimination index of the vehicle group and animals treated
with the higher doses of WIN.
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
Vehicle 1 mg/kg WIN 3 mg/kg WIN 5 mg/kg WIN
Dis
crim
inati
on
In
dex
*

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 42
2.4 Discussion
In this study, activation of cannabinoid receptors dose-dependently decreased the
exploration time towards objects, whether novel or familiar, in adult rats. However, this was
significant only in the 5 mg/kg WIN-treated animals compared to vehicle. When a novel
object was introduced, vehicle-treated animals spent significantly more time exploring the
novel object, while WIN-treated animals did not. The discrimination index set a distinction
between the measures of exploration, motor activity, and novelty-preference as this index
controls for the general effects of WIN on the exploration of familiar as well as novel objects.
As shown in Figure 2.4, only the lowest dose of 1 mg/kg WIN specifically reduced the
discrimination index, indicating that the impairment in object recognition was only at the
lowest dose. Moreover, no significant changes in the total exploration time in the vehicle- and
1, 3 mg/kg WIN-treated groups further indicates that novelty-seeking behaviour and motor
activity are differentially altered by administration of WIN. Therefore, an apparent deficit in
object recognition at a high dose of WIN (i.e. 5 mg/kg) may be compromised as a result of a
general decrease in exploration that affects familiar as well as novel objects. The results of the
present study are consistent with those reported by others in which the effective cannabinoid
drug dose for producing object recognition memory deficits ranges from 0.3 to 1.2 mg/kg
(Schneider and Koch, 2003; O‟Shea et al., 2006; Schneider et al., 2008).
The results of the present study confirm that the adverse effects of CB1 receptor agonists
on object recognition are not restricted to juveniles, and are supported by similar results
reported by others in adult rats (Schneider and Koch, 2002; O‟Shea et al., 2005; Schneider et
al., 2008). Thus the present study adds to a growing body of evidence that object recognition
in adult rats is significantly affected by WIN, despite well-established findings that
behavioural and cognitive deficits seen following CB1 receptor agonist treatment are more
pronounced in pubertal than in adult rats (Schneider and Koch, 2003; O‟Shea et al., 2004;
Cha et al., 2006; Quinn et al., 2008).
Another confirmational finding of the current study is that a single WIN treatment is
sufficiently potent to induce deficits in object recognition in adult rats. Although numerous
studies have used chronic injections of cannabinoid receptor agonists such as 9-THC (Quinn
et al., 2008), WIN (Schneider and Koch, 2003, 2005, 2007; Schneider et al., 2008), or CP-
55,940 (O‟Shea et al., 2004, 2006), only a small number of studies have used acute treatment
with cannabinoid receptor agonists to determine their effect on object recognition memory in

Chapter 2: Cannabinoids and Object Recognition Memory in Rats 43
rats (Schneider and Koch, 2002; Schneider et al., 2008; Fox et al., 2009). Furthermore, a
study by Schneider et al. showed that repeated administration of WIN in adult rats did not
induce any permanent alterations in the discrimination index, object recognition and object
discrimination, whereas acute WIN treatment showed a significant reduction (Schneider et al.,
2008). Since animals have shown the ability to develop tolerance to cannabinoid drugs upon
repeated exposure (see Gonzalez et al., 2005 for review), it is advantageous that single WIN
treatment can induce recognition memory impairments in adult rats.
Object recognition memory is often attributed to the perirhinal cortex and CB1 receptors
are known to be expressed in high densities in this brain region (Liu et al., 2003a). However,
numerous studies have shown that direct administration of cannabinoids into the hippocampus
impaired object recognition memory (Barna et al., 2007; Clarke et al., 2008; Suenaga and
Ichitani, 2008). Furthermore, hippocampal lesion studies have also shown impairment in
object recognition memory, suggesting that the hippocampus also plays a role in the object
recognition memory (Clark et al., 2000; Zola et al., 2000; Broadbent et al., 2004, 2009).
Overall, the results from this investigation suggest that 1 mg/kg WIN is sufficient to produce
cognitive deficits in healthy adult rats. Therefore, whether or not the administration of this
drug will influence spatial memory deficits in BVD rats can now be investigated, and is
described in the next Chapter of this thesis.

44
Chapter 3
The Effect of Cannabinoids on Spatial Memory Following
Bilateral Vestibular Deafferentation in Rats

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 45
3.1 Introduction
In the previous Chapter, it was revealed that 1 mg/kg WIN is sufficient to produce
cognitive deficits in healthy adult rats. Therefore, the effect of this dose of WIN on the
performance of BVD rats in a spatial memory task was investigated in the present study. The
administration of cannabinoid drugs to the BVD animals will address the issue of whether or
not the endocannabinoid system is involved in the pathophysiology of the cognitive
impairments that are observed following bilateral vestibular damage.
Rodents, like many other small animals, must be able to forage for food in their home
ranges while minimising the risk of predation. For successful foraging, animals must
remember their home location and/or keep track of their movements and position in relation
to the home base. The ability to navigate by using external (allothetic) cues, such as visual,
auditory, and magnetic cues, is referred to as piloting. This ability has been shown to exist in
mammals (Sutherland and Dyck, 1984; Collett, 1987; Schenk, 1987), birds (Vander Wall,
1982; Cheng, 1988; 1989), and insects (Collett and Land 1975; Cartwright and Collett, 1982,
1983; Gould, 1987). Although the use of external cues allows animals to navigate accurately,
a single strategy is insufficient in the unpredictable nature of environments. In other words, if
the animal is exposed to a novel or unfamiliar environment where external cues are either
absent or in conflict with previous experience, then the animal has to rely on self-movement
cues, to return to the point where movement originated.
It is a long-standing concept that the vestibular apparatus of vertebrates might serve as
the prime sensing element for animal navigation. In the early 1960s, Schmidt-Koenig, (1965)
defined navigation as an animal‟s ability to maintain or establish reference to a goal other than
through recognition of known landmarks. Indeed navigation requires the information of
present position, direction and magnitude of motion with respect to other points of reference
(Fernandez and Macomber, 1962). Furthermore, Maaswinkel and Whishaw (1999) suggested
that there is a hierarchy in the use of sensory cues during spatial navigation, at least in rats.
Visual cues are used primarily, and then olfactory cues, then other self-movement cues. Other
cues that provide information about self-motion are referred to as idiothetic cues, which
include vestibular information, proprioceptive information, sensory flow, and efferent copies
of motor commands.
The navigational strategy that uses idiothetic cues is known as „dead reckoning‟ or „path

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 46
integration‟ and is colloquially called „sense of direction‟. Particularly important in dead
reckoning is the information from the vestibular system, as it provides the concepts of
horizontality and verticality and allows the detection of changes in acceleration of the body.
The linear and angular acceleration signals from the vestibular system are critical self-
movement cues as they allow the computation of current location and direction in space
relative to a starting location. This is hugely advantageous for an animal, because it means
that the animal can return directly to its home base, even following a complex outward trip.
Directional estimation based on self-movement cues during a foraging task has been shown to
be impaired when the vestibular system is damaged (Wallace et al., 2002c; Zheng et al., 2006,
2009b). While it has been suggested that rats appear to preferentially use allothetic cues,
(primarily visual), over idiothetic cues, many cues can be used flexibly and concurrently
(Maaswinkel and Whishaw, 1999).
Numerous electrophysiological studies provide evidence for the contribution of the
vestibular system to spatial guidance (Sharp et al., 1995; Stackman and Taube, 1997;
Stackman et al., 2002; Russell et al., 2003b, 2006; van der Meer et al., 2007; Bassett et al,
2007; see Smith et al., 2005a, b for reviews). Dead reckoning was first suggested by Darwin
(1873) as a form of navigation and it has been demonstrated in many controlled laboratory
conditions by removing allothetic cues from a testing situation (Etienne et al., 1986;
Maaswinkel et al., 1999; Wallace et al., 2002b; Hines and Whishaw, 2005; Zheng et al.,
2009b). Moreover, a number of animal behavioural studies have demonstrated that vestibular-
lesioned rats are highly dependent upon allothetic cues when performing in a spatial
navigation task, and their ability to utilise dead reckoning is impaired (Stackman and Herbert,
2002; Wallace et al., 2002c; Zheng et al., 2006, 2009b). This reliance on allothetic cues,
especially visual cues, has also been shown in vestibular-deficient patients, where only minor
navigational deficits were observed in the light condition, but marked impairments in spatial
navigation were observed in the dark or when patients were blind-folded (Heimbrand et al.,
1991; Pozzo et al., 1991; Brookes et al., 1993).
In order to effectively assess the role of vestibular information in spatial navigation, it is
important to use a task in which the dead reckoning calculation is made immediately
following the completion of a circuitous outward trip, where each trial is different. Rats have
a natural tendency to collect food and carry it back to a refuge for consumption. This unique
characteristic has been shown to be a robust means by which to dissociate the use of allothetic
and idiothetic cues when foraging in a visually rich environment. First developed by Whishaw

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 47
and Tomie (1997), the foraging task has been used widely to assess animals‟ exploratory
behaviour and their navigational strategies. The task involves a rat leaving a home base and
foraging for a hidden food pellet on a large open circular table. The location of the food pellet
changes between trials, therefore, the circuitous outward searching paths made by a rat will
also change in length, duration, and complexity, depending upon how quickly the food is
found. Rats display different food-handling behaviour toward different sizes of food pellet
(Whishaw et al., 1990). Therefore, the size of the food pellet has to be sufficiently large so
that the rat will carry it back home to eat it, rather than eating it on the table (Whishaw et al.,
1990, 1995). The home base is positioned at the periphery of the table and is hidden
underneath the surface so that it is indistinguishable from seven other holes. Therefore, each
trip is unique with respect to direction and distance. For an accurate return to the home base, a
rat must know the location of the home base in relation to visual cues learned from previous
trials, or else calculate the home direction from its own self movement cues, and thus generate
a direct homeward route. Starting the animals from novel locations and performing the task in
darkness are the versatile features of the foraging task that allow the assessment of which
method is preferentially used when navigating. If a rat returns to an old home location, it is an
indication that it used allothetic cues, mainly visual cues, to guide itself home. However, if a
rat chooses the novel location, then that is an indication that it disregarded the previous
experience and responded to idiothetic cues derived from its just-completed trip.
As previously described, both the vestibular and endocannabinoid systems play a role in
spatial memory functions in animals and humans (see sections 1.1.4 Vestibular-Hippocampal
Connection and 1.2.3 The Role of the Endocannabinoid System in Cognitive Function in
Chapter 1). While Zheng and others have shown that BVD animals have spatial memory
impairment at 5-7 months following BVD surgery in a foraging task (Zheng et al., 2009b),
whether this effect of BVD on spatial navigation is permanent or not has never been
investigated. Furthermore, although the impairment in spatial learning and memory following
cannabinoid receptor agonist treatment has been thoroughly analysed using the classic spatial
memory tasks such as the radial arm maze and the Morris water maze, these tasks cannot
separate the use of self-movement cues from the allothetic cues used by animals. Given that
both the administration of cannabinoid receptor agonists (Lichtman et al., 1995; Robinson et
al., 2003, 2007; Wegener et al., 2008; Yim et al., 2008; Wise et al., 2009) and damage to the
vestibular system (Russell et al., 2003a; Zheng et al., 2006, 2009b) impair animals‟ spatial
memory, the present study aimed to investigate whether BVD-induced impairment in spatial
navigation may be influenced by the administration of a CB1/CB2 receptor agonist, WIN

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 48
55212-2 (WIN) and/or a CB1 receptor inverse agonist, AM251.

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 49
3.2 Materials and Methods
3.2.1 Animals
Thirty-two adult male Wistar rats were used, each weighing 250 – 300 g at the time of
surgery. All animals received either the sham or BVD surgery 14 months prior to the
behavioural testing. However, 4 animals were lost due to aging before the behavioural testing
commenced. The animals were randomly divided into four treatment groups: (1) sham surgery
with vehicle control (n = 8); (2) sham with WIN (n = 7); (3) BVD with vehicle control (n = 6);
(4) BVD with WIN (n = 7). The animals were food deprived to 85% of their normal feeding
weight before and during the behavioural testing. The animals‟ body weight was measured
and recorded every day. Large oat and wheat honey cereal loops were used as food rewards
during behavioural testing. The size of the food reward used in the current study was
validated in previous studies (Zheng et al., 2006, 2009b). After each day of testing, the
animals were given supplementary laboratory rodent food in their home cage in order to
maintain body weight. The necks of rats were spray-painted black (Donaghys Industries Ltd,
Christchurch, New Zealand) to enable tracking and analysis of their movement using the
custom-made software (RatTracker, designed and programmed by the Departmental
Technician, Mr. Kevin Markham).
3.2.2 Peripheral Vestibular Lesion Surgery
In the rat, the surgical lesion of the peripheral vestibular apparatus is a complicated
procedure due to its anatomical location in the inner ear. Moreover, the stapedial artery across
the oval window beneath the stapes, sets further restrictions on the procedure. Because the
vestibular apparatus is accessed via the cochlea, lesions of the cochlea are unavoidable. As a
result, auditory function is completely lost in BVD surgery, in addition to vestibular function.
The surgery was conducted under a general anaesthetic of ketamine hydrochloride (760
g/kg, s.c.), medetomidine hydrochloride (300g/kg, s.c.) and atropine sulfate (80g/kg,
s.c.). The wound margin was anaesthesised locally with xylocaine (5 mg/wound margin, s.c.).
Under microscopic control, the tympanic membrane was exposed using a retro-auricular
approach and the tympanic membrane, malleus, and incus were removed. The stapedial artery
was cauterised and the horizontal and the anterior semicircular canal ampullae drilled open.
The contents of the canal ampullae and the utricle and saccule were then aspirated, and the
temporal bone was sealed with dental cement. Carprofen (5 mg/kg, s.c.) was used for post-

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 50
operative analgesia and atipamezole hydrochloride (5 mg/kg, s.c.) was used to reverse the
effect of medetomidine hydrochloride. Using temporal bone histology, our previous studies
have shown that the BVD surgical procedure produces a complete and permanent lesion of
the vestibular labyrinth with no damage beyond the temporal bone (Zheng et al., 2006).
Sham surgery consisted of exposing the temporal bone and removing the tympanic
membrane without producing a vestibular lesion. This procedure provided a partial auditory
control that involved damage to the tympanic membrane only, with no other surgical trauma.
All other procedures such as anaesthesia and recovery were the same for the sham as for the
lesioned animals.
3.2.3 Drugs
The cannabinoid CB1/CB2 receptor agonist, WIN 55212-2 („WIN‟), and the cannabinoid
CB1 receptor inverse agonist, AM251, were both purchased from Tocris Bioscience (Bristol,
UK). AM251 is chemically similar to SR141617A except that it has a 4-iodophenyl group
instead of 4-chlorphenyl in SR141617A, with a similar potency to SR141716A in tissue
homogenate binding assays (Gatley et al., 1998). The half-lives of WIN and AM251 in rat are
approximately 3 and 22 hrs, respectively (McLaughlin et al., 2003; Agu et al., 2006). WIN
was dissolved in dimethyl sulfoxide (DMSO, Merck) and diluted with saline (0.9% sodium
chloride) in a 1:1 ratio (DMSO : saline) yielding a final concentration of 1 mg/mL, whereas
AM251 was dissolved in a 75% DMSO solution in saline at a concentration of 1 mg/mL. The
drugs were administered s.c. at doses of 1 and 2 mg/kg for WIN and 3 mg/kg for AM251. The
doses utilised were determined based on the previous studies (for WIN, see Chapter 2; Baek
et al., 2009; for AM251, Rodgers et al., 2005; Arenos et al., 2006; Chambers et al., 2006;
Nawata et al., 2010). WIN or vehicle was administered once daily for 21 days. Even though
there may be differences in the effects of WIN under conditions of single versus multiple
injections, the initial effect of WIN on day 1 would essentially be the same as giving a single
injection. The higher 2 mg/kg dose was used when 1 mg/kg did not appear to have a
substantial effect on performance in the foraging task. The vehicle solution was prepared in a
similar manner, with the exception that the drug was omitted.
3.2.4 Foraging Task Apparatus
The apparatus was similar to those used by other researchers and in previous studies in

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 51
our research group (Whishaw and Tomie, 1997; Maaswinkel et al., 1999; Wallace et al.,
2002c; Zheng et al., 2006, 2008b). The apparatus consisted of a 140 cm diameter circular
wooden white table that was elevated 105 cm above the floor (Figure 3.1). Eight 10 cm
diameter holes were located at equal distances around the perimeter of the table, centered 10.5
cm from the table‟s edge, which served as potential home bases for the rats. The table was
mounted on a central bearing such that the table could be rotated between animals. A smaller
circular table (32 cm in diameter) was fixed in the centre of the large table so that it remained
stable when the large table was rotated. This setup was to ensure that the potential odour cues
left on the table could be displaced. These characteristics of the apparatus allowed the home
cage location to be either fixed or variable in relation to the room. A home cage was placed
beneath one of the eight holes from which a rat could climb out onto the table. A metal sheet
was inserted at the bottom of the other seven holes to prevent accidental falls by BVD rats.
Black fabric was placed on top of the metal sheet in order to prevent reflection from the lights
directly above the table, or from the infrared light in the dark condition. The 8 holes had an
identical appearance when viewed from the table surface. A 5.5 cm high transparent perspex
edging was also fitted around the table to prevent falls. Twenty-three food cups (4 cm in
diameter and 1 cm in height) were evenly distributed on the surface of the table.
The apparatus was located in a test room. In this room, many visual cues were available
which included pictures on the wall, the door, and furniture such as cabinets and the computer.
A disc (195 cm in diameter) with a centre bearing was mounted on the ceiling, 130 cm above
the table. An opaque curtain was hung on this disc so that the table could be enclosed from all
visible light during dark conditions. The room lights remained off for both light and dark
conditions. In the light conditions, the apparatus and the room were illuminated by three disc
lights, which were positioned on the disc, 120o apart from each other. In the dark conditions,
the disc lights were turned off and an infrared light source, located inside the curtain, was
used for visualisation of a rat‟s movement through the camera. Rats are unable to see infrared
light (Neitz and Jacobs, 1986). An infrared camera was placed above the centre of the table to
record the movements of the animals under both light and dark conditions. A speaker playing
constant white noise was also mounted above the centre of the table. This was used to
eliminate any possible polarised auditory cues which may affect the performance of the
animals (Rossier et al., 2000; Watanabe and Yoshida, 2007).

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 52
Rotatable
table
32 cm
Fixed
platform
10.5 cm 10 cm
Food cup
Hole
Transparent
edging
Ceiling
disc
130 cm
195 cm
10 cm
10 cm
Lights
Home cage
140 cm
105 cm
Ceiling
Floor
Figure 3.1
The foraging task apparatus. (a) A side view of the apparatus. The home cage was hidden
beneath the surface of the circular table. The lights and white noise generator were located on
the ceiling disc above the table. (b) Dorsal view of the table with 8 potential exit holes around
the periphery. The smaller circular table (dashed line) was fixed in the centre of the larger table,
allowing the larger table to be rotated. The positions of 23 food cups are indicated by open
circles (not drawn to scale).
a)
b)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 53
3.2.5 Foraging Task Procedure
Pre-Training
Before the commencement of the formal tests, the rats were trained until they met the
criterion, which was to retrieve 4 food pellets per 10 minute session. All of the food cups were
baited for the first 2-3 days of the pre-training period. The number of baited cups was
gradually decreased on subsequent days until only 1 food cup was baited per trial. A trial was
defined as an exit from the home cage and a return to the cage with a food pellet. On some
occasions, well-trained rats failed to return to the home cage with a food pellet. Such
responses were classified as a failed trial. The home cage was located beneath the same hole
and at the same location in relation to the room throughout the pre-training. During the pre-
training, the rats learned to climb out of the home cage, search for the food pellet on the table,
and carry it back to the cage. After the rat retrieved a food pellet and returned to the home
cage, a new food pellet was placed in one of the food cups while the rat ate. Some of the rats
initially attempted to eat the food on the table surface rather than carrying it back to the home
cage. They were discouraged from doing so by taking away the pellet or poking them gently
every time they began to eat. All rats quickly learned to discontinue this behaviour and carried
the pellet to the home cage. Pre-training was completed when the rat‟s performance was
stabilised to the 4 search and retrieval trials in succession. The room lights were turned off,
the ceiling frame lights were turned on, and the curtain surrounding the table was drawn back.
Light Probe Trial
In principle, when rats navigate, they can use allothetic cues, idiothetic cues, a
combination of both types, or interchange between cues (Maaswinkel and Whishaw, 1999).
Therefore, in order to determine which cues rats primarily use in light, a probe trial was given
after the completion of pre-training. Throughout the pre-training, the animals were trained
from a single starting location. However, in the light probe trial, the home location was
changed to a novel position, which was located 90o from that in the pre-training. All other
room settings remained exactly the same as in the pre-training phase. Thirty minutes prior to
the trial, each rat was given either the vehicle control or 1 mg/kg WIN (s.c.). Before each rat
began the trial, the foraging table was cleaned with disinfectant to remove any surface cues.
Dark Training
Following the light probe trial, the rats were given 1 day (4 trials) of „normal‟ training in
light where the home cage was positioned at the same location as during the pre-training trials.
The rats did not receive any drug treatment on the „normal‟ training day. After a day of

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 54
„normal‟ training, the rats were then tested in the dark with the curtain surrounding the table.
The training lasted for 21 days. Each rat was subjected to 1 trial per day, in which the position
of the home cage was changed and a different food cup was baited each day. The table was
cleaned with disinfectant between animals. Each animal was administered either vehicle
control or WIN, 30 minutes prior to each trial throughout dark training. One mg/kg WIN (s.c.)
was administered from day 1 to 10, and then the dose was increased to 2 mg/kg from day 11
to 20, due to the apparent lack of effect of the 1 mg/kg dose (described in Results section). On
day 21, all of the animals received AM251 (3 mg/kg, s.c.) 15 minutes before the vehicle
control or WIN injection to determine whether the effect of WIN could be blocked by a single
injection of the cannabinoid receptor inverse agonist.
3.2.6 Data Acquisition
Animals were tested in the foraging task 14 months following BVD surgery. The
number of sessions to reach the foraging task criterion (the retrieval of 4 food pellets per
session per day; Rubino et al., 2009a, b) was recorded in the pre-training phase. Each rat‟s
foraging trip was divided into two segments: searching and homing. The path length (cm), trip
duration (sec) and velocity (cm/sec) for both the searching and homing trips were obtained. In
addition, the first home choice, the second home choice, and the number of choices made
before reaching home (number of errors), and the initial heading angle (o; the direction that
the animal turned immediately after finding food to return home, see detailed description
below), were also obtained from the homeward trip segment. During the light probe trial, the
number of visits made to the old home was also recorded. The number of animals that
completed the task on days 1, 10, 11, and 20 of the dark training was also recorded (Rubino et
al., 2009a, b). These days were chosen as they marked either the first or the last day of WIN
administration at 1 mg/kg or 2 mg/kg. Each exploratory trip was analysed using DVD
playback displayed on a PC and using custom-coded path-tracing software (Figure 3.2). The
sampling rate of the path tracing software was 20 frames/sec.
To measure the initial heading angle, the image constructed by the custom tracking
software was transferred to Adobe Photoshop and enlarged. From this image, the initial
heading angle was measured by using a protractor, where 0o was defined as the most direct
angle possible toward the correct home from the baited food-cup. The angle was measured in
a counter-clockwise direction from a point where the animal had travelled 15 cm from the
baited food cup on the foraging table (Figure 3.3).

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 55
Figure 3.2
The display window of the custom-coded path tracing software on a PC. (a) A window showing
the current camera view of the foraging table. (b) A window that played the DVD recordings of
the animals’ movement for path tracking. (c) Control panel for setting the frame capture rate
and also to start and end the path tracking. (d) An example of the tracing image constructed for
each animal. The initial heading angle was measured from this image.
Figure 3.3
Schematic diagram of measuring the initial heading angle, derived from measuring the angle
between the line of the shortest return route to home (green line) and the line constructed from
the food cup to the heading point where the animal had travelled 15 cm along the homeward
path (red line) on the foraging table.
a) b)
c)
d)
Homeward path
Food
Heading angle
Home 15 cm

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 56
3.2.7 Statistical Analysis
Statistical analyses were performed using various statistical packages including SPSS
17, Oriana 2, Prism 5, and Minitab 15. Anderson-Darling and Levene‟s tests were used to
ensure that the parametric assumptions of normality and homogeneity of variance,
respectively, were not violated. Where assumptions were found to be violated, a natural log
(ln) or square-root data transformation was applied and the data were retested for normality
and equal variance (Rice, 2007). The number of animals in each group varied slightly
between analyses as animals that did not complete the task could not be included in the
analysis.
The number of sessions needed to reach the foraging task criterion was analysed using a
non-parametric Mann-Whitney U test, as the data showed a non-normal distribution which
could not be resolved by data transformation. An area under the curve (AUC) calculation was
used to extract information that was contained in repeated measurements over time (Pruessner
et al., 2003). The AUC values of the pre-training searching and homing time were used to
perform a one-way ANOVA. All of the light probe trial data were analysed using two-way
ANOVAs, except the home choice data which were analysed using circular statistics.
The dark training data analyses for the searching and homing distance, time, velocity,
and the number of errors were performed using a linear mixed model (LMM) analysis using
SPSS 17. The LMM was chosen as an alternative to the general linear model (GLM) with
multi-level ANOVAs, as the data were measured repeatedly, were heavily correlated, and had
unequal samples sizes (Rice, 2007). LMM was performed using a restricted maximum
likelihood procedure, and the most appropriate covariance matrix structure was chosen based
on the smallest Schwarz‟s Bayesian Criterion (Kutner et al., 2005). The AUC calculation was
used to extract information that was contained in repeated measurements over time (Pruessner
et al., 2003). The AUC calculation was essentially an integration of the repeatedly measured
data. After calculating AUC values in Prism 5, three-way ANOVAs were carried out using
SPSS 17 with surgery, treatment, and dose as the factors for each of the dependent variables:
searching and homing distance, time, velocity, and number of errors. For the dose effect, the
AUC values were blocked into 1 and 2 mg/kg WIN treatment.
The analysis of the initial heading angle was conducted using circular statistics and the
Oriana 2 statistics program. A significant Rayleigh‟s test (P < 0.05) suggested that the data
were not uniformly distributed and were clustered around a mean direction (Zar, 1999). The

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 57
percentage of animals that headed to a mean direction was also calculated and was presented
as tendency to a mean direction. The data for the number of sessions to reach criterion were
cube-root transformed to fulfill the assumption of normality and were analysed using a two
sample t-test (SPSS 17). Chi-square analysis was employed to analyse the number of animals
that completed the task (SPSS 17).
Simple and multiple stepwise regression analyses were carried out using Minitab 15 to
determine whether or not the memory impairment could be predicted from the motor activity
of animals, especially in the BVD animals. For the simple regression analysis, searching
velocity AUC was chosen as a predictor variable because it was a measure of hyperactivity,
yet independent from any measure of velocity on homing that might be related to memory.
The AUC for the number of errors was chosen as an index of animals‟ memory and served as
a dependent variable. For the multiple regression analyses, the independent variables were
searching and homing distance, time, and velocity. AUC values were used in these analyses,
as the AUC calculation standardised the units, making the data comparable. An R2 or adjusted
R2 value, (for simple and multiple regression, respectively), indicated how well the regression
explained the data. The closer the R2 value to 1, the better the model predicted the number of
errors from the independent variables.
The data from the light probe trial and from AM251 treatment (day 21), were analysed
using the GLM two-way ANOVAs using SPSS 17.

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 58
3.3 Results
3.3.1 General Behavioural Observations
In general, both the sham and the BVD rats quickly learned to forage for food and
return to the home base. The rat usually poked its head out of the hole a few times before
making its exit. It exited by pulling itself up with its forepaws and pushing with the hind paws.
Once on the table, both the sham and the BVD rats showed a random search for food on the
table. However, the BVD animals were more hyperactive than the sham animals and showed
typical characteristics of BVD animals, such as head weaving, circling, and some of them,
walking backwards. Once a rat found the food pellet, it grasped it in its mouth and set off for
home. The same number of sham animals as BVD animals tried to eat on the table, but they
all quickly learned not to do so. On their return, some of the BVD animals accidentally fell
into the refuge rather than entering stably as all of the sham animals did. Individual rats took
20-30 sec to eat the food before making another foraging trip. More BVD animals exited
home and returned without finding a food pellet (a failed trial) than sham animals. However,
in general, the BVD animals were as motivated as the sham animals to search for food and
were able to carry the food back home to eat.
3.3.2 Pre-Training
Number of Sessions to Reach the Foraging Task Criterion
Although the BVD animals had to be trained for a longer period of time to reach the
foraging task criterion, it was not significantly different (P = 0.290) from the sham animals
(Figure 3.4).
Figure 3.4
Number of pre-training sessions required for the sham (n = 15) and BVD (n = 13) animals to
reach the foraging task criterion (retrieval of 4 food pellets per 10 min session per day). The
data are represented as mean + SEM.

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 59
Searching and Homing Time
The BVD animals had a significantly longer searching time compared to the sham
animals until session 15 (F(1,19) = 6.18; P = 0.022; Figure 3.5b). However, on the last two days
of the pre-training, there was no significant difference between the two groups in their
searching time (F(1,20) = 0.036; P = 0.851; Figure 3.5c), suggesting that the BVD animals
could search for the food as effectively as the sham animals. Regression analysis indicated
that there was no significant change in homing time across the last six days of the pre-training
sessions, which indicated that the animals had a stable performance (R2 = 0.056; Figure 3.5d).
However, the BVD animals showed a significantly longer homing time compared to sham
controls (F(1,20) = 61.60; P = 0.000; Figure 3.5e).
SEARCHING
HOMING
Figure 3.5
Searching (a) and homing (d) time of the sham and BVD animals in the last 6 days of the pre-
training. (b) and (c) The AUC of the searching time in sessions 12-15 and 16-17, respectively
and (e) The AUC of the homing time. Data are represented as mean + SEM.
a) b)
c)
d) e)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 60
3.3.3 Light Probe Trial
First and Second Home Choices
Upon finding the food, most of the sham animals went directly to the old home location
where they had been trained during the pre-training phase, but the BVD animals did not.
Circular statistical analysis on the first home choice demonstrated that sham animals had a
significant preference for the old home location, whereas BVD animals did not (Rayleigh test;
r = 0.76; P = 3.31 x 10-4
for sham; r = 0.098; P = 0.930 for BVD). However, once they
discovered that the home cage was no longer at the old home location, the animals carried on
searching for home. The second home choice was randomly distributed for both sham and
BVD animals (r = 0.45; P = 0.113 for sham; r = 0.51; P = 0.287 for BVD; Figure 3.6). There
was no significant difference between the WIN- and vehicle-treated animals for the first or
second home choice.
Figure 3.6
First and second home choices for the light probe trial. The large circle represents the foraging
table and eight circles on the periphery of the table represent the potential home bases. The
hatched circle represents the old home location, and the closed one represents the novel home
location. The coloured circles outside the table represent the individual animals in various
groups. The direction and the length of the arrow in the middle of the table represents the
mean direction and tendency for the first and second home choices for sham (green) and BVD
(purple) animals. The black dotted line represents the direction for the correct home (that is, 0o
or 360o), and it serves as a reference to show animals’ deviation on their home choices.
1st Choice
Old home New home
2nd
Choice
SHAM-Veh SHAM-WIN BVD-Veh BVD-WIN

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 61
Searching and Homing Distance, Time, Velocity
The effects of surgery and drug treatment were not statistically significant and there
were no significant interactions (Figure 3.7).
Figure 3.7
Foraging task parameters displayed by sham-vehicle, sham-WIN, BVD-vehicle, and BVD-WIN
animals in the light probe trial. (a), (b), (c) Searching distance, time, velocity, respectively; (d),
(e), (f) homing distance, time, velocity, respectively. The data are represented as mean + SEM.
SEARCHING HOMING
a) d)
b)
c)
e)
f)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 62
b)
Searching and Homing Velocity Comparison
Both the sham and BVD animals had a significantly higher homing velocity than
searching velocity during the light probe trial (three-way ANOVA; F(1,31) = 24.36; P = 0.000;
Figure 3.8a). There were no statistically significant effects for surgery (F(1,31) = 0.64; P =
0.429), drug treatment (F(1,31) = 2.10; P = 0.157; Figure 3.8b), or of any of the interactions
between factors.
Figure 3.8
The velocity comparison between searching and homing in the light probe trial. (a) The
difference in searching and homing velocities between the sham and the BVD animals; (b) the
effect of vehicle and WIN treatment on the sham and the BVD animals’ velocity. Data are
represented as mean + SEM.
d)
a)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 63
Number of Visits to Old Home
Before reaching the correct home, sham animals made significantly more visits to the
old home compared to the BVD animals (two-way ANOVA; F(1,16) = 6.81; P = 0.019).
However, there was neither a significant drug treatment effect (F(1,16) = 0.47; P = 0.501) nor a
significant interaction between the surgery and the drug treatment (F(1,16) = 0.57; P = 0.463;
Figure 3.9a).
Number of Errors
There was no significant difference between the sham and the BVD animals in the
number of errors made before reaching the correct home (two-way ANOVA; F(1,15) = 0.94; P
= 0.348). Furthermore, there was no significant drug treatment effect or interaction (F(1,15) =
0.006; P = 0.939 for drug treatment; F(1,15) = 0.082; P = 0.778 for surgery x drug treatment
interaction; Figure 3.9b).
Figure 3.9
(a) The average number of visits to the old home, and (b) the average number of errors made
before reaching the correct home by sham-vehicle, sham-WIN, BVD-vehicle, and BVD-WIN
animals in the light probe trial. The data are represented as mean + SEM.
a) b)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 64
3.3.4 Dark Training
Searching Distance
BVD animals travelled a significantly longer distance in searching for food compared to
the sham animals (linear mixed model (LMM) analysis; P = 0.000). However, there was no
significant drug effect (P = 0.628; Figure 3.10a). The three-way ANOVA on the AUC values
further confirmed the above results (F(1,78) = 24.59; P = 0.000 for surgery; F(1,78) = 2.30; P =
0.134 for drug treatment; Figure 3.10b) and showed a significant dose effect, where both the
sham and BVD animals had a significantly shorter searching distance in the 2 mg/kg WIN
dose than in the 1 mg/kg (F(1,78) = 3.99; P = 0.049; Figure 3.10b). There was also a significant
interaction between surgery, treatment and dose (F(1,78) = 5.06; P = 0.027; Figures 3.10c, d),
but no other significant interactions.
Figure 3.10
(a) Linear graph of the searching distance (cm) for different treatment groups during the dark
training with the data reflecting a block of two sessions. (b), (c), and (d) The AUC of the
searching distance for the sham and BVD animals to show the effect of surgery, dose and the
interactions between surgery, treatment, and dose. Data are represented as mean + SEM.
a)
b) c) d)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 65
Searching Time
Neither surgery nor drug treatment had any significant effect on searching time in the
dark training (LMM analysis; P = 0.202 for surgery; P = 0.365 for drug treatment; Figure
3.11a). The three-way ANOVA on the AUC values further confirmed these results (F(1,78) =
1.10; P = 0.298 for surgery; F(1,78) = 2.93 ; P = 0.091 for drug treatment; Figure 3.11b, c).
There was no significant dose effect (F(1,78) = 0.91; P = 0.343) or any significant interactions
between factors.
Figure 3.11
(a) Linear graph of the searching time (sec) for different treatment groups during the dark
training with the data reflecting a block of two sessions. (b) and (c) The AUC of the searching
time showing the effect of surgery, treatment, and dose. Data are represented as mean + SEM.
a)
b) c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 66
Searching Velocity
BVD animals had a significantly higher searching velocity compared to sham animals
(LMM analysis; P = 0.000). However, there was no significant drug effect (P = 0.082; Figure
3.12a). The three-way ANOVA on the AUC values confirmed the above results (F(1,78) = 8.33;
P = 0.005 for surgery; Figure 3.12b; F(1,78) = 2.84; P = 0.096 for drug treatment; Figure 3.12c).
There was a significant dose effect, where animals‟ searching velocity was significantly
decreased with 2 mg/kg WIN treatment compared to 1 mg/kg (F(1,78) = 10.35; P = 0.002).
There were no significant interactions between surgery, drug treatment, and dose.
Figure 3.12
(a) Linear graph of the searching velocity (cm/sec) for different treatment groups during the
dark training with the data reflecting a block of two sessions. (b) and (c) The AUC of the
searching velocity showing the effect of surgery, treatment, and dose. Data are represented as
mean + SEM.
a)
b) c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 67
Homeward Path
The homeward path taken by the sham and the BVD animals in the dark training clearly
showed that the sham animals took the most direct route home (Figure 3.13a), whereas the
BVD animals did not (Figure 3.13b). The BVD animals made directionless movements across
the entire foraging table; some visited every other hole until they serendipitously found the
home.
Figure 3.13
An example of the homeward path taken by a (a) sham and a (b) BVD rat in the dark training.
The large circle represents the foraging table. The small black circle in the middle of the table
represents the location of the food pellet, and the other black circle near the periphery
represents the home location.
a) Sham b) BVD

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 68
Homing Distance
The BVD animals travelled a significantly longer distance to return home compared to
sham animals (LMM analysis; P = 0.000). However, there was no significant drug effect (P =
0.188; Figure 3.14a). The three-way ANOVA on the AUC values further confirmed these
results (F(1,78) = 50.03; P = 0.000 for surgery; Figure 3.14b; F(1,78) = 0.88; P = 0.353 for drug
treatment; Figure 3.14c) and showed a significant dose effect, where animals had a
significantly shorter homing distance for the higher WIN dose (F(1,78) = 28.37; P = 0.000;
Figure 3.14c). Furthermore, there was a significant interaction between surgery and dose,
indicating that the BVD animals had a shorter homing distance for 2 mg/kg WIN, while the
sham animals did not show any change (F(1,78) = 18.74; P = 0.000; Figure 3.14b). There were
no other significant interactions.
Figure 3.14
(a) Linear graph of the homing distance (cm) for different treatment groups during the dark
training with the data reflecting a block of two sessions. (b) and (c) The AUC of the homing
distance showing the effect of surgery, treatment, and dose. Data are represented as mean +
SEM.
a)
b) c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 69
Homing Time
The BVD animals had a significantly longer homing time compared to the sham
animals (LMM analysis; P = 0.000). However, there was no significant drug effect (P = 0.991;
Figure 3.15a). The three-way ANOVA on the AUC values further confirmed these results
(F(1,78) = 17.18; P = 0.000 for surgery; Figure 3.15b; F(1,78) = 2.32; P = 0.132 for drug
treatment; Figure 3.15c) and showed a significant dose effect (F(1,78) = 20.42; P = 0.000;
Figure 3.15c). Furthermore, there was a significant interaction between surgery and dose,
indicating that the BVD animals had a shorter homing time for 2 mg/kg WIN, while the sham
animals did not show any change (F(1,78) = 10.33; P = 0.002; Figure 3.15b). There were no
other significant interactions.
Figure 3.15
(a) Linear graph of the homing time (sec) for different treatment groups during the dark training
with the data reflecting a block of two sessions. (b) and (c) The AUC of the homing time
showing the effect of surgery, treatment, and dose. Data are represented as mean + SEM.
b) c)
a)
b) c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 70
Homing Velocity
The BVD animals had a significantly higher homing velocity compared to sham
animals (LMM analysis; P = 0.000), but there was no significant drug effect (P = 0.680;
Figure 3.16a). However, the three-way ANOVA on the AUC values showed no significant
surgery or dose effect (F(1,78) = 3.71; P = 0.058 for surgery; F(1,78) = 2.82; P = 0.097 for dose;
Figure 3.16b). Furthermore, WIN treatment significantly decreased animals‟ homing velocity
(F(1,78) = 5.25; P = 0.025; Figure 3.16c) There were no significant interactions between the
surgery, treatment, and dose.
Figure 3.16
(a) Linear graph of the homing velocity (cm/sec) for different treatment groups during the dark
training with the data reflecting a block of two sessions. (b) and (c) The AUC of the homing
velocity showing the effect of surgery, treatment, and dose. Data are represented as mean +
SEM.
a)
b) c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 71
Searching and Homing Velocity Comparison
Both the sham and BVD animals had a significantly higher homing velocity than
searching velocity in the dark training (3-way ANOVA; F(1,20) = 34.05; P = 0.000; Figure
3.17a). In addition, the BVD animals had a significantly higher overall velocity compared to
the sham animals (F(1,20) = 7.20; P = 0.009). However, WIN treatment significantly reduced
animals‟ velocity (F(1,20) = 8.37; P = 0.005; Figure 3.17b). However, there were no significant
interactions between factors.
Figure 3.17
The velocity comparison between searching and homing during the dark training. (a) The
difference in searching and homing velocities between the sham and the BVD animals; (b) The
effect of vehicle and WIN treatment on the sham and the BVD animals’ velocity. Data are
represented as mean + SEM.
a) b)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 72
Heading angle - Sham0
90
180
270 50 50
50
50
40 40
40
40
30 30
30
30
20 20
20
20
10 10
10
10
Heading angle - BVD0
90
180
270 15 15
15
15
12.5 12.5
12.5
12.5
10 10
10
10
7.5 7.5
7.5
7.5
5 5
5
5
2.5 2.5
2.5
2.5
Initial Heading Angles
When they found the food, the sham animals made a clear head turn toward the correct
home and went home in almost a straight path. A Rayleigh test on the initial heading angle
confirmed that there was a clear orientation towards home for the sham animals (r = 0.60; P <
1 x 10-12
; Figure 3.18). However, the BVD animals‟ heading angles were uniformly
distributed around 360o (r = 0.072; P = 0.498; Figure 3.18). Furthermore, the WIN treatment
did not affect sham animals‟ clear orientation toward home nor did it have any effect on BVD
animals (Figure 3.19).
SHAM
BVD
Figure 3.18
Rose diagram showing the initial heading angles of the sham and BVD animals for the dark
training. The mean vector is indicated by the black line and 95% confidence interval (C.I.) for
the mean is indicated by the line extending either side. The 95% C.I. values for the BVD animals
were unreliable due to low concentration of vectors, hence the red line. The inner circles
(dotted line) indicate the number of observations for the given vectors (blue triangles).

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 73
1 mg/kg WIN treatment
SH
AM
– V
ehic
le
SH
AM
– W
IN
BV
D –
Veh
icle
BV
D –
WIN
2 mg/kg WIN treatment
SH
AM
– V
ehic
le
SH
AM
– W
IN
BV
D –
Veh
icle
BV
D –
WIN
Figure 3.19
Initial heading angles of animals in different treatment groups for 1 and 2 mg/kg WIN treatment.
SHAM-Veh0
90
180
270 25 25
25
25
20 20
20
20
15 15
15
15
10 10
10
10
5 5
5
5
SHAM-WIN0
90
180
270 25 25
25
25
20 20
20
20
15 15
15
15
10 10
10
10
5 5
5
5
BVD-Veh0
90
180
270 10 10
10
10
7.5 7.5
7.5
7.5
5 5
5
5
2.5 2.5
2.5
2.5
BVD-WIN0
90
180
270 6 6
6
6
5 5
5
5
4 4
4
4
3 3
3
3
2 2
2
2
1 1
1
1
SHAM-Veh0
90
180
270 25 25
25
25
20 20
20
20
15 15
15
15
10 10
10
10
5 5
5
5
SHAM-WIN0
90
180
270 15 15
15
15
12.5 12.5
12.5
12.5
10 10
10
10
7.5 7.5
7.5
7.5
5 5
5
5
2.5 2.5
2.5
2.5
BVD-Veh0
90
180
270 10 10
10
10
8 8
8
8
6 6
6
6
4 4
4
4
2 2
2
2
BVD-WIN0
90
180
270 3 3
3
3
2 2
2
2
1 1
1
1

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 74
First and Second Home Choices
Most of the sham animals made their home choice the correct home even on the first
day in the dark (r = 0.73; P = 9.97 x 10-5
). The sham animals had a significant preference for
the correct home on their first and second home choice throughout the dark training, whereas
the BVD animals did not (Figure 3.20). Both the sham and BVD animals‟ home choices were
not affected by the WIN treatment. However, vehicle-treated BVD animals had a preference
for an incorrect hole on their first home choice in the 2 mg/kg WIN treatment period.
Figure 3.20
First and second home choices of the sham and BVD animals with 1 and 2 mg/kg WIN
treatment in the dark training. The large circle represents the foraging table and eight circles
on the periphery of the table represent the potential home bases. The closed circle represents
the correct home location. The direction and the length of the arrows in the middle of the table
represent the mean direction and tendency of the home choices for sham (green) and BVD
(purple) animals. The black dotted line represents the direction for the correct home (that is, 0o
or 360o), which served as a reference to show animals’ deviation on their home choices.
2n
d choice
2 mg/kg WIN treatment
1st ch
oice
1 mg/kg WIN treatment

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 75
Number of Errors
The BVD animals made significantly more errors before reaching the correct home
compared to the sham animals (LMM analysis; P = 0.000; Figure 3.21a; three-way ANOVA;
F(1,78) = 78.64; P = 0.000; Figure 3.21b). Although there was no significant drug effect overall
(LMM analysis; P = 0.267; Figure 3.21a; three-way ANOVA; F(1,78) = 1.59; P = 0.211; Figure
3.21c, d), WIN treatment significantly decreased the number of errors made by the BVD
animals, (F(1,78) = 6.37; P = 0.014; Figure 3.21c), particularly at the higher dose (F(1,78) = 8.02;
P = 0.006; Figure 3.21b). In fact, animals made significantly fewer errors overall with 2
mg/kg WIN treatment compared to 1 mg/kg (F(1,78) = 8.87; P = 0.004; Figure 3.21d). There
were no other significant interactions.
Figure 3.21
(a) Linear graph of the number of errors made before reaching the correct home by animals in
different treatment groups with the data reflecting a block of two sessions. (b), (c), and (d) The
AUC of the number of errors for the sham and BVD animals to show the effect of surgery, drug
treatment, dose, and their interactions. Data are represented as mean + SEM.
a)
b) d)
c)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 76
Number of Animals that Completed the Task
There were no significant surgery or drug treatment effects for the number of animals
that completed the foraging task throughout the dark training (Figure 3.22).
Figure 3.22
The number of animals that completed the task during the dark training.
Simple Regression Analysis
A simple regression analysis was performed on the AUC for the number of errors made
(dependent variable) against the AUC for the searching velocity (predictor variable) of the
vehicle-treated sham and BVD animals. It showed that the number of errors made by the
animals could not be predicted from their searching velocities (R2 = 0.004 for sham, Figure
3.23a; R2 = 0.115 for BVD, Figure 3.23b). When WIN-treated animals were included in the
analysis, no statistically reliable prediction could be drawn (R2 = 0.104 for sham, Figure 3.23c;
R2 = 0.017 for BVD, Figure 3.23d).
Multiple Stepwise Regression Analysis
The multiple stepwise regression analysis was done to determine whether the AUC for
the number of errors made by sham or BVD animals could be predicted from the AUCs for
various predictor variables, such as searching and homing distance, time, or velocity.
Although the stepwise process revealed that the homing time and velocity predicted the
number of errors made, the adjusted R2 values were too low to make this prediction
statistically reliable (adjusted R2 = 0.453 for sham; adjusted R
2 = 0.435 for BVD).

Vehicle group only WIN group included
SH
AM
200190180170160150140130120
16
14
12
10
8
6
4
2
0
AUC searching velocity
AU
C n
o. o
f e
rro
rs
25020015010050
32.5
30.0
27.5
25.0
22.5
20.0
17.5
15.0
AUC searching velocity
AU
C n
o. o
f e
rro
rs
BV
D
2001751501251007550
16
14
12
10
8
6
4
2
0
AUC searching velocity
AU
C n
o. o
f e
rro
rs
25020015010050
35
30
25
20
15
10
AUC searching velocityA
UC
no
. o
f e
rro
rs
Figure 3.23
Simple regression analysis performed to predict the number of errors made by vehicle-treated (a) sham and (b) BVD animals or by (c) all of the sham and
(d) the BVD animals from their searching velocities. The AUC values were used to perform the regression analysis.
b)
a) c)
d)
R2 = 0.004
R2 = 0.115
R2 = 0.104
R2 = 0.017

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 78
3.3.5 AM251 Treatment
Searching and Homing Distance, Time, Velocity
When treated with AM251, the BVD animals had significantly higher searching and
homing velocities compared to the sham animals (two-way ANOVA; F(1,14) = 18.12; P = 0.001
for searching; F(1,14) = 5.25; P = 0.038 for homing; Figure 3.24). There were no statistically
significant differences in any other measurements taken.
Searching and Homing Velocity Comparison
Both the sham and BVD animals had a significantly higher homing velocity than
searching velocity (three-way ANOVA; F(1,28) = 31.72; P = 0.000; Figure 3.25a). Furthermore,
the BVD animals had a significantly higher overall velocity than the sham animals (F(1,28) =
23.82; P = 0.000; Figure 3.25a, b). However, there was no significant drug treatment effect
(F(1,28) = 0.099; P = 0.756) or any interactions.
Figure 3.25
The velocity comparison between searching and homing with AM251 treatment on day 21 of
the dark training. (a) The difference in searching and homing velocities between the sham and
the BVD animals; (b) The effect of drug treatments on the sham and the BVD animals’ velocity.
Data are represented as mean + SEM.
a) b)

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 79
Figure 3.24
Foraging task parameters displayed by sham-vehicle, sham-WIN, BVD-vehicle, and BVD-WIN
animals with AM251 treatment on day 21 of the dark training. (a), (b), (c) Searching distance,
time, velocity, respectively; (d), (e), (f) homing distance, time, velocity, respectively. The data
are represented as mean + SEM.
SEARCHING
a)
b)
c)
d)
e)
f)
HOMING

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 80
First and Second Home Choices
AM251-WIN treated animals showed a significant preference for the correct home on
their first home choice (Rayleigh test; r = 0.68; P = 0.054), while AM251-vehicle treated
animals did not (r = 0.27; P = 0.425). However, AM251-vehicle treated animals showed a
significant preference for the correct home on their second choice (r = 0.64; P = 0.021). The
number of AM251-WIN treated animals that made the second choice was too small (n = 2) to
perform a statistical analysis. The sham animals had a significant preference for the correct
home base on their first home choice, whereas BVD animals did not (r = 0.63; P = 0.01 for
sham; r = 0.25; P = 0.653 for BVD; Figure 3.26). The second home choice was randomly
distributed for both sham and BVD animals (r = 0.57; P = 0.199 for sham; r = 0.48; P = 0.255
for BVD; Figure 3.26).
Figure 3.26
First and second home choices of the BVD and sham animals on day 21 of the dark training
with the treatment of AM251 prior to vehicle or WIN administration. The large circle represents
the foraging table and eight circles on the periphery of the table represent the potential home
bases. The closed circle represents the correct home location. The coloured circles outside the
table represent the individual animals in various groups. The direction and the length of the
arrows in the middle of the table represent the mean direction and tendency of the home
choices for sham (green) and BVD (purple) animals. The black dotted line represents the
direction for the correct home (that is, 0o or 360
o), which served as a reference to show animals’
deviation on their home choices.
1st Choice
SHAM-Veh SHAM-WIN BVD-Veh BVD-WIN
2nd
Choice

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 81
Initial Heading Angles
AM251-vehicle treated animals showed a clear direction (Rayleigh test; r = 0.61; P =
0.008), while AM251-WIN treated animals did not (r = 0.492; P = 0.243; Figure 3.27).
Furthermore, the sham animals had a clear direction towards home, while the BVD animals‟
heading angles were uniformly distributed around 360o, indicating that the BVD animals had
no sense of direction when they turned to head back home (r = 0.73; P = 0.001 for sham; r =
0.419; P = 0.304 for BVD).
Figure 3.27
Initial heading angles of (a) vehicle- or (b) WIN-treated animals on day 21 of the dark training
with the treatment of AM251 prior to vehicle or WIN administration.
Number of Errors
The BVD animals made significantly more errors before reaching the correct home
compared to the sham animals (two-way ANOVA; F(1,14) = 4.69; P = 0.048). However, there
was no significant drug treatment effect (F(1,14) = 0.758; P = 0.399) or surgery x drug
treatment interaction (F(1,14) = 0.132; P = 0.722; Figure 3.28).
Figure 3.28
The graph showing the average number of errors made before reaching the correct home by
the sham-vehicle, sham-WIN, BVD-vehicle, and BVD-WIN animals on day 21 of the dark training.
The data are represented as mean + SEM.
a) AM251-Vehicle b) AM251-WIN

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 82
3.4 Discussion
This study had two major aims. The first was to investigate the effects of BVD on
spatial memory using the foraging task in animals that were at a time point long after the
surgical lesions – 14 months. The second was to determine whether a cannabinoid receptor
agonist, WIN, could exacerbate the spatial memory deficits caused by BVD, and whether an
inverse agonist, AM251, could reduce this effect. Aside from determining whether the
memory deficits could be modulated by cannabinoid drugs, such results would suggest
whether cannabinoid receptors, in the hippocampus and elsewhere, might be involved in the
effects of BVD on spatial memory.
This study provided strong evidence that the cognitive impairment due to the vestibular
damage is highly likely to be permanent. More specifically, at 14 months post-operation,
BVD animals were unable to use allothetic cues during their navigation to return home and
their spatial memory impairment was more severe in the dark than in the light condition.
Overall, these impairments appear to be more severe than at 5-7 months post-operation
(Zheng et al., 2009b) and administration of a cannabinoid receptor agonist showed a
complicated effect on these deficits.
There was no indication that the BVD animals were restricted in their movement or
showed any impairment in their ability to acquire the task. It has been reported that BVD
animals are more reluctant and hesitant to leave the home base compared to control animals in
the foraging task (Wallace et al., 2002c; Zheng et al., 2006, 2009b). One might argue that this
reluctance is due to the motor impairments that follow the vestibular lesions. However, this
could not have been the case because previous studies of BVD rats reported that not only are
they not limited in movement, but they are hyperactive (Russell et al., 2003a; Goddard et al.,
2008a; Zheng et al., 2006, 2007, 2008, 2009b), and also any vestibulo-spinal reflex deficits
due to BVD that might restrict animals‟ movement would have compensated to some extent
by the time of their performance (Smith and Curthoys, 1989).
Furthermore, in the pre-training, there was also no significant difference between the
sham and BVD animals in the searching distance. Indeed, BVD animals in the present study
showed hyperactivity, which was indicated by a higher locomotor velocity compared to sham
animals. Moreover, there was no significant difference in the searching distance between the
sham and BVD animals in the light probe trial. These results confirm that the BVD animals

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 83
were capable of generating the necessary motor activity to perform food searching. Further
evidence that BVD surgery left animals‟ ability to learn the task intact was provided in the
pre-training sessions where there was no significant difference between BVD and sham
animals in the number of trials to reach the criterion or in the searching time on the last 2 days
of training. This was consistent with a previous study where rats with bilateral sodium
arsanilate-induced vestibular lesions reached the criterion in an equivalent number of trials in
a spatial navigation task (Stackman and Herbert, 2002). Although BVD animals were capable
of homing during the pre-training, they did so with a significantly longer time. This was in
agreement with the previous study when the animals were tested at 5-7 months post-operation
(Zheng et al., 2009b). A question raised from this result was whether the BVD animals are
still able to use allothetic cues to guide them home as they did in the previous study.
Therefore, a light probe trial was given during which the home location was changed to a
novel position.
Effects of BVD on Spatial Navigation In Light
In the light probe trial, when the home choice and the number of visits to the old home
were analysed, the performance of the sham and the BVD animals was distinctly different.
The sham animals showed a significant preference for the old home location on their first
home choice, whereas the BVD animals did not. The sham animals‟ preference for the old
home location on their first choice was in agreement with previous studies (Maaswinkel and
Whishaw, 1999; Whishaw et al., 2001; Wallace et al., 2002c; Zheng et al., 2006, 2009b), and
suggests that visual cues were used during their piloting.
The BVD animals‟ random first home choice was a novel finding from the present study.
Both the Wallace et al. (2002c) and Zheng et al. (2009b) studies reported that BVD animals
showed a preference for the old home on their first choice. There are two possible
explanations for the lack of old home preference in the light probe trial by the BVD rats in the
current study. First, the extent of vestibular damage in the animals in the Wallace et al. (2002c)
study was variable as BVD was achieved by intratympanic injection of sodium arsanilate.
This magnitude of variation was indicated by the numerical scores given from 0 (no vestibular
damage) to 9 (complete vestibular damage). Although chemical ablation of the peripheral
vestibular labyrinth is often used to create a vestibular lesion in a rat (Ossenkopp and
Hargreaves, 1993; Stackman et al., 2002), this procedure has been shown to be unreliable, as
it often results in incomplete lesions (Jensen, 1983; Saxon et al., 2001). Therefore, it is
possible that the extent of vestibular damage may have accounted for the difference in the

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 84
homing choice. However, Zheng et al. (2009b) performed surgical ablation, which would
have caused complete vestibular damage without much variation. As the same surgical
technique was used in the present study, the absence of old home preference in the 14 months
post-BVD animals suggests that it was not just the degree of the vestibular damage that
contributed to the spatial deficits in the BVD animals in light, but also the length of post-
operation time. In other words, it is possible that the BVD animals‟ spatial deficits became
worse over time. In the Wallace et al. (2002c) study, BVD rats were tested only 2 weeks
following the surgery, while Zheng et al. (2009b) tested their animals at 5-7 months after the
surgery. In the present study, the animals were tested 14 months following the surgery.
Therefore, BVD animals‟ inability to use available allothetic cues and execute piloting as their
navigating strategy in the present study might be an indication that the BVD animals‟
cognitive deficits became worse between 7 and 14 months post-BVD.
The second home choice in the light probe trial was randomly distributed for both the
sham and the BVD animals. This result was consistent with the study by Zheng et al. (2009b).
However, the sham animals‟ random second home choice was contradictory to studies by
Wallace et al. and Whishaw et al. (Wallace et al. 2002c; Whishaw and Tomie, 1997; Whishaw
and Maaswinkel, 1998; Maaswinkel et al., 1999). In their studies, almost all of the control rats
proceeded directly to the new location after finding that the home was no longer present at the
old location. However, Zheng et al. (2009b) showed that when allothetic cues are available, it
is not common for the animals to switch to idiothetic cues even if they might have been
collected during navigation. This inflexibility in switching between the allothetic and
idiothetic cues is further evidenced by sham animals‟ persistent visits to the old home, since
the animals should have made their second home choice the new home location if idiothetic
cues had been used.
One possible explanation for the lack of ability in using allothetic cues in BVD animals
in light is that animals had poor vision and/or the lighting set up around the foraging table
may have been insufficient for them to utilise the available allothetic cues to navigate
themselves back to the previously correct home. However, sham animals‟ obvious preference
for the old home on their first home choice and persistent visits to the old home suggests that
the sham animals at least had adequate vision and the lighting around the foraging table was
sufficient. For the BVD animals, it is possible that they were still experiencing oscillopsia due
to permanent loss of their vestibulo-ocular reflexes (Smith and Curthoys, 1989), which may
have contributed to their poor performance in light. Nevertheless, it is curious that at 5-7

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 85
months post-BVD, the only deficit observed in light was a significantly longer homing time
(Zheng et al., 2009b). At 14 months post-BVD, it might be expected that the animals would
be better adapted to oscillopsia than at 5-7 months, and hence use visual cues more effectively.
Again, this suggests that BVD animals are more impaired in the foraging task at 14 months
than at 5-7 months post-operation and that their spatial memory deteriorates over time.
The random first home choice and the small number of visits to the old home by the
BVD animals in the light probe trial suggests that the BVD animals were more susceptible to
spatial reference memory impairment than the sham animals. This deficit might have been
due to the natural aging of the animals. Indeed, age-related deficits in spatial memory have
been previously observed in rats (Caprioli et al., 1990, 1991; Frick et al., 1995; Markowska,
1999; see Barnes, 2004 for review). In these studies, spatial reference memory deficits were
observed at the age of 17-18 months, but spatial working memory was not impaired until 24-
28 months of age. The animals in the present study were 16 months old when they were tested
in the foraging task. However, if the spatial reference memory deficit was due to aging, the
sham animals should have demonstrated the deficits as well. But this was not the case – sham
animals had a clear preference for the old home on their first home choice and they visited the
old home several times to ensure that the home was no longer there. Only the BVD animals
showed the impairment in their home choice and in the number of visits, which were
indications of the spatial reference memory deficits.
Another possible explanation for BVD animals‟ spatial impairment even in light is that
BVD animals might not have actually learnt the home location during pre-training, but
reached home by chance alone. Not only the probability of finding the home by chance would
have been equal between the sham and the BVD animals, the comparison of searching and
homing velocity in the light probe trial demonstrated that BVD animals were actively homing.
It has been shown that animals return to the home base with a greater velocity than in their
outward trip (Eilam and Golani, 1989; Drai et al., 2000; Whishaw et al., 2001; Wallace et al.,
2002b; Wallace and Whishaw, 2003). In the present study, both the sham and BVD animals
showed a significantly higher homing velocity than searching velocity, which suggested that
the BVD rats were actively homing, but possibly were unaware of the precise location of their
home base.
BVD animals‟ impaired spatial memory in light suggests that they were unable to
collect the information about the home location from vision alone. The next dominant sensory

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 86
cue in the rat is the olfactory cue (Maaswinkel and Whishaw, 1999). Since the BVD animals
would not have had any self movement cues from the vestibular system, it is possible that
they relied on olfactory cues more substantially than the sham animals. There has been
considerable debate concerning the use of olfactory cues by animals in maze tasks (Means et
al., 1971, 1992; Wallace et al., 2002a), and the contribution of olfactory cues in path
integration has previously been shown to be negligible (Maaswinkel and Whishaw, 1999;
Whishaw and Gorny, 1999; Whishaw et al., 2001; Wallace and Whishaw, 2003). Furthermore,
the results from the present study suggest that it is unlikely that homing was guided by
olfactory cues in the light probe trial. There are several reasons for this. First, the rats‟
foraging excursions were circuitous and therefore, it seemed improbable that there was a
direct track that guided animals‟ homeward movement. Second, if the animals used olfactory
cues, (for example, the odour of the cage that they had just left) or followed their own odour
trail, they would have returned to the new home location and not to the old location. However,
there was a clear preference for the old home by the sham animals. Not only did the BVD
animals did not show any preference for the old home in the light probe trial, they also did not
make their first home choice the new home, suggesting that the BVD animals did not use
olfactory cues as their dominant cue. This leads to the third reason. It has been shown that rats
following odour trails or a scented track travel much more slowly (Wallace and Whishaw,
2003). Comparison of searching and homing velocity between BVD and sham animals
demonstrated that homing velocity was higher than searching velocity not only in the sham
animals but also in the BVD animals, which suggests that the BVD animals were not
following any odour trails. Fourth, in a previous study in which animals were trained to
follow a scented string that leads to a food pellet in the dark, after the retrieval, the animals
took a relatively direct route back home without following the scented string which would
also have led them home (Whishaw and Gorny, 1999; Whishaw et al., 2001). These results
suggest that in the absence of visual cues, the self-motion cue is pre-eminent over the
olfactory cues. Finally, tracking rats have a distinct posture in which the nose is down and the
back is arched (Whishaw and Gorny, 1999; Wallace et al., 2002a), and this posture was not
observed by the homing sham or BVD rats in the current study. For these reasons, it can be
concluded that it is unlikely that the animals used olfactory cues for guidance in the foraging
task.
As chemical or surgical lesions of the vestibular labyrinth usually involve damage to the
cochlea as well, it is possible that any cognitive effects of the lesions are partly due to hearing
loss. For example, auditory stimulation, including noise trauma, has been reported to affect

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 87
place cell function (Sakurai, 1990, 1994; Goble et al., 2009). For this reason, sham animals
were included in the present study as partial auditory controls. Although some sound will still
be transmitted to the cochlea in the sham animals, these animals have consistently performed
significantly better in cognitive tasks than animals with vestibular lesions (Zheng et al., 2006,
2007, 2008, 2009a, b). This suggests that hearing loss is not the major cause of the spatial
memory deficits in animals subjected to bilateral vestibular lesions. Furthermore, animal
studies, using different kinds of aminoglycosides (i.e. streptomycin and neomycin) with
different toxicities for the auditory and vestibular hair cells, have shown that the effects of
auditory and vestibular lesions on learning and memory are different (Schaeppi et al., 1991).
In a radial arm maze task, rats treated with streptomycin, which lesioned the auditory and the
vestibular systems, exhibited impaired working memory; however, rats treated with neomycin,
which lesioned only the auditory system, did not (Schaeppi et al., 1991). Recently, it was
shown that rats subjected to acoustic trauma do not exhibit deficits in the Morris water maze
task, suggesting that their spatial memory is intact and that the auditory damage does not have
any profound effect on cogntion (unpublished observations).
Effects of BVD on Spatial Navigation In Darkness
The spatial navigation ability was most affected by the vestibular damage when the only
source of information available was idiothetic cues, that is, the vestibular and/or the
proprioceptive information. In darkness, the BVD animals had a significantly longer homing
distance and homing time compared to the sham animals. The BVD animals‟ circuitous
homing path and sham animals‟ direct path clearly indicated that the BVD animals had no
strategy for finding the correct home after retrieving the food, whereas the sham animals were
able to use self-movement cues to calculate the shortest route home. This was consistent with
previous publications reporting that vestibular information is required for path integration
(Wallace et al., 2002c; Zheng et al., 2006, 2009b). It could be argued that BVD rats‟ inability
to use a path integration strategy on their homeward trip in the dark training was due to their
longer searching distance compared to the sham rats, resulting in reduced homing accuracy. In
previous studies, similar searching distance, time, and velocity were observed for both sham
and BVD animals in the dark (Wallace et al., 2002c; Zheng et al., 2009b), suggesting that the
BVD animals were able to search for food effectively. However, this ability was impaired in
BVD animals at 14 months post-operation as evidenced by a significantly longer searching
distance. Nevertheless, such impairment seems to be compromised by the higher searching
velocity in BVD animals and as a result the searching time was not significantly different
between the sham and BVD rats, which suggested that both groups spent an equal amount of

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 88
time searching for food. Although the time spent searching for food could affect the homing
accuracy in the dark, searching distance is probably more important in terms of correctly
tracking the idiothetic cues on the searching path. This, together with the evidence that BVD
animals at 5-7 months were impaired in homing in the dark, but could still search for food
effectively, suggests that the BVD animals‟ longer searching distance observed in the present
study may account for their impairment in homing in the dark to some extent.
The impairment in BVD animals‟ ability in using self-movement cues to find their way
back home in darkness was further demonstrated by their initial heading angles and the home
choices. Throughout the dark training, the BVD animals‟ initial heading angles were
uniformly distributed around 360o, while the sham animals‟ initial heading angles were
clustered around the correct home base. Consistent with their initial heading angles, BVD
animals‟ first and second home choices were either random or clustered around an incorrect
location, whereas the sham animals showed a clear preference for the correct home location.
These results indicated that the BVD animals did not have any sense of direction. Similar
findings were reported for 5-7 months post-operative animals by Zheng et al. (2009b).
The impairment of homing in darkness was unlikely to be due to a lack of motivation in
the BVD animals. Although the BVD animals had significantly higher velocities in both
searching and homing, the comparison of the two types of velocities showed that the
hyperactivity of the BVD animals was not the same across the two trip segments; searching
and homing. The homing velocity was significantly higher than the searching velocity for
both the sham and BVD animals, which suggests that the BVD animals were actively homing
over and above their hyperactivity. This implies that any impairment in homing would not
have been due to BVD animals‟ hyperactivity or lack of motivation. Furthermore, although it
took the BVD animals longer to find home, they did poke their head into every potential
home location they passed by, inevitably resulting in a significantly higher number of errors
compared to the sham animals. This again suggests that the BVD animals were motivated to
return home without being aware of the exact home location. Similar results were observed in
the 5-7 months post-BVD animals in the Zheng et al. (2009b) study.
The hyperactivity observed in the BVD animals could potentially prevent them from
homing correctly by generating unnecessary movements or resulting in an attention deficit.
Indeed, BVD animals have been found to exhibit impairment in a 5-choice serial reaction time
task, which is used extensively to investigate animals‟ attention and such deficits could be part

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 89
of the explanation for the spatial memory deficits (Zheng, et al., 2009a). Nevertheless, the
simple regression analysis proved, for the first time, that the BVD animals‟ hyperactivity
could not predict their performance in the foraging task, as the number of errors (a measure of
animal‟s memory) could not be predicted from their searching velocity (a measure of
hyperactivity). This novel finding is of significance because BVD animals‟ hyperactivity has
been reported not only in surgically damaged BVD rats (Zheng et al., 2007, 2008, 2009b), but
also in sodium arsanilate-treated BVD animals (Ossenkopp et al., 1990; Porter et al., 1990;
Stackman and Herbert, 2002; Wallace 2002c). Moreover, as none of the measurements
(searching and homing distance, time, and velocity) could reliably predict the degree of
memory impairment in the animals, it seems that the BVD animals‟ motor activity is not
related to their performance in the spatial memory task. Taking these results together, it is
unlikely that hyperactivity accounted for the impairment in homing by BVD rats.
This study has shown, for the first time that rats with BVD do not recover from spatial
memory deficits even at very long time intervals following the surgery, such as 14 months.
From the estimated average life expectancies of rats (2.5 years, Baker et al., 1979) and
humans (85 years, Olshansky et al., 1990; Manton et al., 1991), 14 months post-BVD in rats
is calculated to be approximately 40 years for humans. Therefore, the results from the present
study suggest that that spatial memory deficits following vestibular damage may be
permanent.
Effects of Cannabinoid Drugs
The present study was the first to investigate the effect of cannabinoids using the
foraging task, and also was the first to demonstrate the effect of cannabinoids on spatial
learning and memory in BVD animals. It was expected that the administration of the
cannabinoid receptor agonist would exacerbate the observed spatial memory deficits in the
BVD animals as it has been shown to have disruptive effects on spatial learning and memory
in both animals and humans previously (Litchman et al., 1995; Cha et al., 2006; Barna et al.,
2007; Nestor et al., 2008; Pamplona et al., 2008; Suenaga and Ichitani, 2008; Weinstein et al.,
2008; Wise et al., 2009). However, the effect of WIN was more complicated than expected.
There have been concerns about cannabinoid receptor agonists affecting performance in
behavioural tasks by influencing motivation and reward-related behaviour. However, the
effect of cannabinoid receptor agonists on motivation has been inconsistent and contradictory,
where some studies have shown increased motivation (Higgs et al., 2005; Solinas and

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 90
Goldberg, 2005), while others have shown a reduction in motivation (Higgs et al., 2005;
Drews et al., 2005). Nevertheless, in the present study, WIN-treated animals showed no
significant differences in searching distances or searching times compared to the vehicle-
treated group either in light or in darkness, which suggested that WIN treatment did not
interfere with the animals‟ motivation to perform in the foraging task.
In the present study, WIN treatment significantly decreased animals‟ homing velocity
and their overall velocity, which raised a concern about whether the WIN treatment
contributed to the reduction in animals‟ motor activity. Indeed, cannabinoid receptor agonists
have been shown to decrease animals‟ motor activity (McGregor et al., 1996; Darmani, 2001;
Romero et al., 2002; Järbe et al., 2006; Fox et al., 2009). However, there are several reasons
why it is unlikely that the reduction in animals‟ velocity influenced their performance in the
foraging task. First, there was no significant difference between vehicle- and WIN-treated
animals in their searching time, which suggested that WIN-treated animals spent an equal
amount of time searching for food to the vehicle-treated animals. Second, if WIN treatment
reduced animals‟ motor activity, then the homing time should have been longer for WIN-
treated animals than vehicle-treated animals because WIN-treated animals would have moved
more slowly, and therefore, should have taken a longer time to return home. However, WIN
had no significant effect on homing time and in fact homing time was shorter for the higher
dose of WIN, which suggests that WIN treatment did not reduce animals‟ locomotor activity
at the doses used in the present study.
In the dark, the higher dose of WIN significantly reduced homing distance and time, and
the number of errors specifically in BVD animals, which suggested that WIN treatment might
have improved performance in the dark in BVD animals. However, WIN treatment was not
associated with any improvement in the initial heading angle or the first home choice. This
improvement was observed only in BVD animals, which suggested that BVD might have
resulted in changes in the endocannabinoid system in the brain (see Chapter 4). Moreover,
WIN might have led BVD animals to use different strategies other than path integration,
although at this stage it is not clear what these may be. However, given that the higher dose of
WIN significantly reduced homing velocity in animals, WIN treatment might have aided
BVD animals in effective homing by reducing their homing velocity.
There are some possible explanations for the general lack of effect of WIN on spatial
memory in the BVD and sham animals observed in the present study. One obvious

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 91
explanation is that the doses employed were too low. However, it needs to be noted that the
doses were chosen based on an object recognition study that was conducted in order to
determine doses that would impair memory without causing sedation (see Chapter 2; Baek et
al., 2009). Furthermore, based on previous studies (Wallace et al., 2002c; Zheng et al., 2009b),
it was predicted that because BVD rats were likely to exhibit spatial memory deficits, the
animals might be more sensitive to the adverse effects of WIN. For this reason, the animals
were first treated with the 1 mg/kg dose, and then the dose was increased to 2 mg/kg when it
became clear that the lower dose was having little effect, either on sham or BVD animals.
Although it is generally well accepted that cannabinoid receptor agonists impair spatial
memory (Suenaga and Ichitani, 2008; Robbe and Buzsaki, 2009; Wise et al., 2009), their
effects on memory are complex, dose-dependent (Abush and Akirav, 2009) and even memory
enhancement has been reported in some cases (Marchalant et al., 2008; Campolongo et al.,
2009). WIN did not seem to impair spatial memory even in the sham animals, as would be
expected. However, it is unlikely that this was due to the WIN doses being too low, because
the number of errors was actually reduced with the higher dose in darkness. It is conceivable
that the reason why WIN did not exacerbate spatial memory deficits in the BVD animals is
that they were already performing so poorly that the cannabinoid receptor agonist could not
impair this performance further.
To add to the complexity of the results, pre-treatment with AM251 abolished the effect
of WIN on homing time and the number of errors in the dark. Furthermore, animals that had
received AM251 treatment in combination with WIN showed a significant preference for the
correct home on their first home choice while AM251 treatment alone did not, suggesting that
AM251 treatment may impair path integration on its own, but does not have any effect when
given with WIN. Because inverse agonists such as SR141617A and AM251 have a tendency
to produce effects that are opposite to those produced by agonists for the CB1 receptors
(Landsman et al., 1997; Sim-Selly et al., 2001; see Pertwee, 2005a; Bergman et al., 2008 for
reviews), the behavioural consequences of their inverse agonist activity in combination with a
CB1 receptor agonist are difficult to interpret. However, recently, a CB1 receptor antagonist,
AM4113, has been developed (Sink et al., 2008). Although the effects of CB1 receptor
antagonists on animals‟ cognitive function are yet to be determined, future studies should be
carried out using these drugs to further clarify the function of the CB1 receptor and the
involvement of the endocannabinoid system in BVD animals.
An important issue to remember in the present study is that AM251 was given only once,

Chapter 3: Cannabinoids and Spatial Memory in BVD Rats 92
while WIN was administered for 20 days. While many studies have investigated the
consequences of the chronic administration of cannabinoid receptor agonists in animals,
studies involving cannabinoid receptor inverse agonists (AM251 and/or SR141617A) have
mainly focused on their effects in reversing the agonist-induced effects. Interestingly,
Hoffman et al. (2007) showed a significant enhancement of LTP in hippocampal slices taken
from animals that were treated with AM251 for 7 days (2 mg/kg/day, i.p.). However, an acute
exposure of hippocampal slices from drug-naïve rats to AM251 (1 M) did not have any
effect on LTP (Hoffman et al., 2007). The authors postulated that long-term treatment with
cannabinoid receptor inverse agonists may act to improve the mechanisms that support
memory (Hoffman et al., 2007). Based on these results, future studies should use long-term
treatment with AM251 as it might show a memory enhancement effect in BVD animals.
Furthermore, intrahippocampal administration of cannabinoids to the BVD animals should
also be considered for future investigation as it would provide evidence for the area specific
effects of cannabinoids in BVD animals and further clarify the involvement of the
endocannabinoid system in the cognitive deficits caused by bilateral loss of the vestibular
function.
Overall, although there was a lack of effect of cannabinoids, the present study was the
first study to demonstrate a possible connection between vestibular- and cannabinoid-
mediated memory impairment. Since the hippocampus has been shown to undergo plasticity
following BVD (Stackman et al., 2002; Russell et al., 2003b, 2006; Goddard et al., 2008c)
and CB1 receptors are highly expressed in the hippocampus, it is logical to examine CB1
receptor expression in the hippocampus following BVD. Furthermore, given that the density
of CB1 receptor expression is not necessarily indicative of the affinity or efficacy of the
receptor (Breivogel et al., 1997; Rubino et al., 2009a), it is also logical to investigate CB1
receptor binding affinity in BVD animals. These intriguing questions are investigated in the
next Chapter of this thesis.

93
Chapter 4
Changes in CB1 Receptor Density and Affinity Following
Bilateral Vestibular Deafferentation in Rats

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 94
4.1 Introduction
In the previous Chapter, it was revealed that BVD causes significant impairments in
spatial memory in rats, and that the effects of cannabinoid drugs in BVD animals are more
complex than expected. Since CB1 receptors are well known for their neuromodulatory effects
in the brain (see Section 1.2.2 Neuromodulatory Role of the Endocannabinoid System in
Chapter 1; also see Rodriguez de Fonseca et al., 2005; Pertwee, 2006; Breivogel and Sim-
Selley, 2009 for reviews), any changes in CB1 receptor density or affinity following BVD
may provide evidence for the involvement of the endocannabinoid system in brain
dysfunction observed following peripheral vestibular damage. As the hippocampus undergoes
significant synaptic changes following BVD, and high levels of CB1 receptors are found in
this area, the aim of the present study was to determine whether CB1 receptor density and
affinity in the hippocampus would change following BVD in rats. The results from the present
in vitro biochemical assays may provide some insight into the cellular and molecular
mechanisms that may underlie BVD-induced spatial memory deficits.
Although the involvement of the endocannabinoid system in various pathological
conditions has been well established, the primary focus of previous investigations has been on
changes in the number of CB1 receptors. Many believe that the effects of cannabinoids are
determined by the distribution of CB1 receptors in the brain (Herkenham et al., 1991, Jansen
et al., 1992; Sim et al., 1995), presumably assuming that these receptors have a uniform high
affinity, yet the binding affinity of CB1 receptors is not well characterised. Furthermore,
although numerous studies have been published investigating the efficacy of CB1 receptors
using a cannabinoid agonist-stimulated guanylyl 5‟-[-[35
S]thio]-triphosphate ([35
S]GTPS)
binding assay (Selley et al., 1996; Petitet et al., 1997; Breivogel et al., 1997; Vinod et al.,
2005; Castelli et al., 2007; see Childers, 2006 for review), there are very few studies
investigating the affinity of the CB1 receptor in the rat whole brain (Compton et al., 1993;
Hillard et al., 1995; Houtston and Howlett, 1993, 1998; Thomas et al., 1998) and even fewer
studies focusing specifically on the hippocampus (Romero et al., 1995; Gatley et al., 1997;
Castelli et al., 2007; Hill et al., 2010).
It is firmly established that the pharmacological actions of cannabinoids are mediated
through specific cannabinoid receptors (see Pertwee, 2006 for review). The CB1 receptors are
coupled to pertussis toxin-sensitive Gi/o proteins and their biochemical actions are effected by
the activation of these G-proteins (see Section 1.2.1 Cannabinoid Receptors and

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 95
Endocannabinoids in Chapter 1; Howlett et al., 1986; see Demuth and Molleman, 2006 for
review).
The first step in this signal transduction involves the binding of a ligand to a CB1
receptor. The degree to which this receptor-ligand binding occurs is measured as affinity,
expressed in molar units, normally in a nanomolar range (see Pertwee, 2005b for review). The
affinity of the cannabinoid receptor can be determined by displacement assays using tritiated
cannabinoids. The basic theory behind the radioligand binding assay is simple. A membrane
preparation containing the receptor of interest is incubated with a radioligand in conditions
favourable for the ligand to bind to the receptor. Then the radioactivity of the bound ligand is
detected. A saturation binding assay measures the specific binding at equilibrium at various
concentrations of radioligand to determine the receptor density (Bmax) and the affinity of the
receptor for the radioligand (Kd, the dissociation constant). If a single concentration of the
radioligand is used and the concentration of an unlabelled competing ligand is varied, then the
assay is defined as a competitive binding assay and the affinity of the competing ligand (Ki)
can be estimated accordingly. Furthermore, the concentration of the unlabelled ligand required
to block 50% of the specific binding of the radioligand (the IC50) can also be determined from
the competitive binding assay (see Bylund and Toews, 1993 for review).
Several different tritium-labelled ligands for cannabinoid receptors have been used in
previous studies. One of the most commonly used radioligands in these studies is [3H]-CP-
55,940, due to its high and approximately equal affinity for both the CB1 and CB2 receptors
(see Pertwee, 1999; Howlett et al., 2002 for reviews). Moreover, [3H]-CP-55,940 was the
radioligand used in the first cannabinoid receptor binding assay which characterised the CB1
receptors in the rat brain (Devane et al., 1988). Early studies revealed that [3H]-CP-55,940
binding is rapid, saturable, reversible, and has high affinity (Devane et al., 1988; Compton et
al., 1993; Houston and Howlett, 1993; Gatley et al., 1997; Kearn et al., 1999). Its use in
receptor binding assays in membrane preparations and tissue sections has facilitated the
characterisation and localisation of the cannabinoid receptors in the brain and in peripheral
tissues (Hekenham et al., 1991; Compton et al., 1993; Munro et al., 1993). Thus [3H]-CP-
55,940 was chosen for use in the present study.
To date, there has been no study investigating changes in CB1 receptor density, affinity,
or efficacy following BVD. Although a number of studies have investigated changes in the
level of the NMDA receptors (Li et al., 1997; King et al, 2002; Liu et al., 2003b), -amino-3-

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 96
hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Li et al, 1996; King et al,
2002), glucocorticoid receptors (Lindsay et al., 2005; Zhang et al., 2005b), GABA receptors
(Horii et al., 2003; Zhang et al., 2005a), and CB1 receptors (Ashton et al., 2004b) following
vestibular lesions, it has all been following UVD. Given that the effects of UVD and BVD in
animals and in humans are distinctly different (Zheng et al., 2004, 2004, 2006, 2007, 2009b;
Brandt et al., 2005; Hüfner et al., 2007), the changes in receptor properties following BVD
may or may not be similar to what has been observed in animals following UVD.
Since the adverse effects of BVD on the hippocampus appear to be immediate and long-
lasting (results from Chapter 3; Stackman et al., 2002; Russell et al., 2003a, Zheng et al.,
2009b), it might be expected that the changes in CB1 receptor density and affinity would
occur shortly after BVD. The time following BVD at which changes are observed in CB1
receptor density and affinity in the hippocampus may give further indication of how
permanent the effects of BVD may be, and give further insight into interactions between the
vestibular and endocannabinoid systems. Therefore, the present study aimed to determine
hippocampal CB1 receptor density and affinity at 1, 3, and 7 days following BVD by using
western blotting and a [3H]CP-55,940 binding assay, respectively.

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 97
4.2 Materials and Methods
4.2.1 Western Blotting
4.2.1.1 Animals
Thirty-nine naïve male Wistar rats were used, each weighing 250-300 g at the time of
surgery (see Chapter 3, section 3.2.2 for surgery details). There were 3 post-operative time
points: 1, 3, and 7 days. There were 6 sham and 7 BVD animals for each time point.
4.2.1.2 Tissue Collection
All animals were decapitated without anaesthesia at 1, 3, or 7 days after the surgery. The
whole brain was rapidly removed from the skull and immediately placed into ice-cold 0.9%
saline solution. The hippocampus was dissected into 3 sub-regions: CA1, CA3, and dentate
gyrus (DG) under a dissecting microscope. The dissection plate was placed on dry ice in order
to keep the tissue cold. Dissected tissue samples of individual structures were immediately
frozen on dry ice and stored separately at -80o C until use.
4.2.1.3 Tissue Homogenisation
Tissue buffer containing 50 mM Tris buffer (pH 7.6) and complete proteinase inhibitor
(1:25 dilution of stock; Roche) was added to the samples on ice, which were then
homogenised using ultrasonification (Sonic Vibra Cell, John Morris Scientific Ltd.).
Homogenates were centrifuged at 12,000 x g for 10 minutes at 4 o
C (Centrifuge 5810 R,
Eppendorf). Supernatant was collected and 5 L of each sample was taken for the Bradford
assay (see below) to determine the protein concentration.
4.2.1.4 Bradford Assay
Protein concentrations in the supernatant were measured using the Bradford method
(Bradford, 1976) using a Bio-Rad protein assay dye reagent concentrate. Serial dilution of the
bovine serum albumin (BSA) standard solution (1 mg/mL; Sigma) was carried out by
obtaining various concentrations of the standard solutions. These concentrations were: 500,
250, 125, 62.5, 31.25, 15.63, and 7.81 g/mL. Blank solution (0 g/mL) did not contain any
BSA solution. Supernatants of each sample were diluted by a factor of 60 using distilled water

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 98
(dH2O). Standard solution and samples were each loaded in triplicate into a 96-well plate. In
each well, 150 L of dH2O and 40 L of Bradford reagent (Bio-Rad) were added. The plate
was read using a microplate spectrophotometer (Benchmark Plus, Bio-Rad) set to a maximum
wavelength of 595 nm, and the standard curve was generated with the correlation coefficient
of > 0.98. The protein concentrations of the original samples were then calculated and the
sample concentrations were equalised for each hippocampal sub-region using the tissue buffer.
The equalised samples were mixed with the sample loading buffer (125 mM Tris-HCl, pH 6.8;
4% SDS, 20% glycerol, 10% 14.3 M mercaptoethanol, 2 mM EDTA, bromophenol blue
dissolved in dH2O) in a ratio of 1:1, then boiled for 5 minutes. The final concentration for
each sub-region was 3 mg/mL for CA1 and DG and 2 mg/mL for CA3. Samples were stored
at -20o C until use.
4.2.1.5 Western Blot
Gel Preparation
Both resolving and stacking gels were prepared at room temperature. A ten % resolving
gel (10% N,N‟-methylene-bis-acrylamide (Bio-Rad), 0.375 M Tris buffer (pH 8.8; Sigma),
0.1% sodium dodecylsulfate (SDS, Sigma), 5% glycerol (BDH), 1% N,N,N‟,N‟-
tetremethylethylenediamine (TEMED, Bio-Rad) and 0.036% ammonium persulfate (APS,
Bio-Rad)) was used in order to adequately separate the CB1 receptor protein (54 and 63 kDa).
TEMED and APS were added last into the gel mixture as they catalysed the polymerisation
and allowed the gel to set. Immediately after adding TEMED and APS, the gel mixture was
decanted in vertical mini-gel cassettes (Protean II, Bio-Rad). The cassettes consisted of two
glass plates separated by spacers (0.75 mm thick), which were held together by side clamps
and were sealed at the bottom. Methanol was used to flatten the surface of the resolving gel
during setting. Once the resolving gel was set, methanol was discarded and the top of the gel
was washed three times with dH2O before laying the stacking gel (5% N,N‟-methylene-bis-
acrylamide, 0.129 M Tris buffer (pH 6.8), 0.2% SDS, % TEMED, 0.11% APS). A plastic
comb was inserted into the stacking gel layer to create an individual well. The comb was
carefully removed after the stacking gel had completely set, and the cassettes were transferred
into the electrophoresis tank and filled with running buffer (0.3% Tris-Base, 1.44% glycine,
1% SDS w/v in dH2O).
Loading, Electrophoresis and Transfer
Dual colour protein marker (Bio-Rad) was loaded (5 L) in each gel as a molecular

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 99
weight standard and samples were loaded accordingly. The sample loading volume was
adjusted for the CB1 receptor antibody, as different antibodies show different levels of
sensitivity to the same samples. For each region of the hippocampus, 12 g of protein was
loaded in each well. In addition, a control tissue sample used as an internal standard was
loaded on each gel in order to control for the between gel variation.
Once all the samples were loaded, the electrophoresis tank was connected to a 400 W
power supply (PowerPack 3000; Bio-Rad) and the gel was run at 90 V until samples reached
the interface of the stacking and resolving gel, then at 180 V until samples reached the end of
the gel. Gels were removed from the glass plates, trimmed, and laid onto blot paper (7.5 x 10
cm; Bio-Rad). A polyvinylidene fluoride (PVDF; Bio-Rad) membrane, cut to the same size as
the gel, was pre-soaked in absolute methanol and then in transfer buffer at room temperature
(0.3% Tris-Base, 1.44% glycine, 20% absolute methanol) for 5 minutes each to equilibrate
before covering the gel. Each gel and PVDF membrane was sandwiched between blot papers
in a Trans-Blot Cassette (Bio-Rad) and inserted into a Trans-Blot Cell (Bio-Rad). Transfer
buffer was poured over the set up to completely cover the gel sandwich. Blotting took place
overnight at 10 V at room temperature.
Immunoblotting and Detection
Following completion of the transfer, the PVDF membrane was blocked for 6-7 hours at
4o C with blocking solution consisting of 0.1% BSA and 5% non-fat milk dissolved in TTBS
(10% 50 mM Tris buffer saline (TBS) pH 7.6, 0.1% Tween-20). The membrane was then
incubated with the CB1 receptor primary antibody (0.5% non-fat milk in TTBS; 1:1000
dilution, goat polyclonal antibody raised against a peptide mapping at the N-terminus of CB1
of human origin and recommended for detection of CB1 of mouse, rat, and human origin, sc-
10066, Santa Cruz Biotechnology, Inc) overnight at 4o
C. Following primary antibody
incubation, the membrane was washed four times with TTBS, for 5 minutes each time. The
membrane was then incubated with the horseradish peroxidase (HRP)-conjugated donkey
anti-goat secondary antibody (0.5% non-fat milk in TTBS; 1:5000 dilution, Santa Cruz
Biotechnology, Inc) for 4 hours at 4o C. After secondary antibody incubation, the membrane
was washed in TTBS four times, then twice in TBS for 5 minutes each time. The membrane
was lightly dried on tissue paper before immersing it in an enhanced chemiluminescence
(ECL) reagent (Amersham Biosciences) for 1 minute. ECL reagents allow the HRP-
conjugated secondary antibody to illuminate so that the membrane can be developed on a
photosensitive film. Following exposure to the ECL reagent, the membrane was placed in a

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 100
plastic envelope and taped inside a „Hypercassette‟ (Amersham). For immunodetection, all
procedures were carried out in a dark room. A sheet of photosensitive film (Amersham) was
placed on top of the luminescent membrane in the hypercassette. The exposure time of the
film to the membrane varied according to the strength of the signal, however, it was usually 1-
2 minutes. For developing, the film was first immersed in a developing solution (1:5 dilution;
Kodak) for 1 minute. The developing reaction was terminated by immersing the film in a stop
solution (1:25 dilution; Kodak) for 30 seconds. Photo-reactivity of the film was neutralised in
a fix solution (Kodak) for 4 minutes. The film was then rinsed under running tap water and
dried (Fc, JRC-33 air dryer). The molecular weight standards were marked on the film in
order to identify the target protein bands.
Stripping and Re-probing membranes
After developing, the PVDF membrane was submerged in a stripping buffer (20% SDS,
12.5% 0.5 M Tris HCl (pH 6.7), 0.8% -mercaptoethanol, 67.5% dH2O) for 30 minutes at 50o
C with constant agitation. This process was necessary for „stripping‟ off primary and
secondary antibody complexes from the membrane and to allow re-probing of the membrane
with different antibodies. Following stripping, the membrane was rinsed with TTBS four
times (5 minutes per rinse), and kept in TBS at 4o C until use. The membrane was stripped a
maximum of two times.
Blocking Peptide
The common problem with polyclonal antibodies is that non-specific binding of an
antibody (binding to proteins other than the antigen of interest) can sometimes occur.
Therefore, to determine which band was specific for the CB1 receptor protein, a blocking
peptide (sc-10066 P, Santa Cruz Biotechnology, Inc) was used at 1:1 ratio (primary antibody :
blocking peptide). The primary antibody was „neutralised‟ by the blocking peptide, that is, the
antibody was bound to the blocking peptide, and was therefore no longer available to bind to
the epitope present in the protein on the western blot. Thes neutralised antibody was then used
to simultaneously blot a membrane to the antibody alone, and the results were compared.
4.2.1.6 Data Acquisition
Protein Quantification from Western Blot
Developed film was analysed using a Calibrated Imaging Densitometer (Bio-Rad),
PowerPC Mac running OS 9.2 and Quantity One software. For each band, optical volume was

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 101
presented as a product of the frame area (mm2) and optical density (OD; average pixel
intensity per frame). Background volume was also taken for each lane to calculate specific
volume. The optical volume of CB1 receptor protein in each sample was normalised against
the optical volume of the internal standard for between gel comparison.
Normalisation to a „House Keeping‟ Protein
In order to control for any inconsistency in loading samples, „house-keeping‟ proteins
are used as internal controls to check whether the differences in the amount of protein of
interest could be due to an initial difference in the amount of sample loaded. The most
commonly used protein to serve this purpose is -actin, as actin is a cytoskeletal filament that
forms the internal scaffolding of a cell, and therefore its expression is ubiquitous in neuronal
and non-neuronal tissue (Lecuit and Lenne, 2007). Each membrane was therefore blotted for
actin (1:5000 dilution, Santa Cruz Biotechnology, Inc) to normalise the level of
immunolabelling of the CB1 receptor protein to actin, so that any potential variations in
protein loading could be eliminated.
4.2.1.7 Statistical Analysis
Statistical analyses were performed using SPSS 17. A series of two-way analyses of
variance (ANOVAs) followed by Bonferroni post hoc tests were performed in order to
determine whether BVD affected the levels of hippocampal CB1 receptor protein at different
post-operative time points. The data were tested for normality and homogeneity of variance
and were natural log (ln) transformed where necessary.
4.2.2 Radioligand Binding Assay
4.2.2.1 Animals
Forty-five naïve male Wistar rats were used, each weighing 250-300 g at the time of
surgery (see Chapter 3, Section 3.2.2 for surgery details). There were 3 post-operative time
points: 1, 3, and 7 days. For each time point, there were 7 sham and 8 BVD animals.
4.2.2.2 Cannabinoid Ligands
[3H] [1,2-(R)-5]-5-(1,1-dimethyleptyl)-2-[5-hydroxy-2-(3-hydroxy propyl)

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 102
cyclohexyl] phenol ([3H]-CP 55,940) (174.6 Ci/mmol) was purchased from Perkin Elmer Life
Sciences, Inc. WIN 55212-2 (WIN) was purchased from Tocris Bioscience (Bristol, UK). One
hundred mM of WIN (stock solution) was prepared in dimethyl sulfoxide (DMSO, Merck)
and stored at -20o C until use. When preparing the serial dilution solutions, loading buffer (50
mM Tris (pH 7.4), 1 mM EDTA, 3 mM MgCl2, 0.5% BSA) was added to make a final WIN
concentration of 1 mM (working solution).
4.2.2.3 Membrane Preparation
Animals were decapitated without anaesthesia. The whole brain was rapidly removed
from the skull and immediately placed into ice-cold 0.9% saline solution. The hippocampus
was dissected out from the forebrain and collected in 0.32 M of sucrose (Sigma) solution. The
hippocampus was then homogenised in 1.5 mL of 0.32 M sucrose solution using 10 gentle
strokes of a 2 mL hand-held glass teflon homogeniser (Kontes), which was kept cold in ice.
All of the membrane preparation procedures were carried out at 0 – 4° C. The homogenate
was centrifuged for 10 minutes at 1000 x g to remove unbroken cells and blood vessels. The
resulting supernatant was collected and centrifuged at 20,000 x g for 20 minutes, and the
pellet was then re-suspended in ice-cold dH2O and further centrifuged for 20 minutes at 8,000
x g. After centrifugation, a fluffy white-coloured band was obtained at the interface, and a
dark brown-coloured, mitochondria-rich pellet obtained at the bottom of the tube. Both the
supernatant fraction and buffy coat (the pale layer on top of the dark brown mitochondrial
pellet) were then collected and centrifuged for 10 minutes at 48,000 x g. The pellet was re-
suspended in Tris-HCl buffer (50 mM, pH 7.4; Sigma), and then centrifuged for 10 minutes at
48,000 x g. The pellet was immediately frozen on dry ice for 2 minutes in order to eliminate
endogenous ligand (i.e. endocannabinoids) within the membranes. The synaptosomal pellet
was resuspended in Tris-buffer (50 mM, pH 7.4) and centrifuged again at 48,000 x g for 10
minutes. These freezing, re-suspension, and centrifugation steps were repeated once. The
crude membrane receptor preparations were stored at -80o C until use.
The Bradford protein assay was performed in order to determine the protein
concentrations in each sample (see Section 5.2.4). The protein concentration in the samples
were then equalised to 1 mg/mL.

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 103
4.2.2.4 [3H]-CP 55,940 Binding Assay
The [3H]-CP 55,940 binding assay protocol used was modified from Sawant et al.
(2008). The concentration of [3H]-CP 55,940 was selected based on the Kd values of [
3H]-CP
55,940 from previous studies (Hillard et al., 1995; Castelli et al., 2007; Hill et al., 2008,
2010). In the present study, WIN 55212-2, which has been shown to be structurally different
to CP 55,940 (Georgieva et al., 2008), was selected to determine non-specific binding. Bylund
and Toews (1993) recommended that it is best to use a drug that is chemically distinct from
the radioligand, as it reduces the possibility of the unlabelled drug inhibiting „specific‟ but
non-receptor binding sites. Furthermore, the [3H]-CP 55,940 binding assay was carried out
using a whole hippocampus rather than separate sub-regions of the hippocampus as it would
not provide practical sample volume to run the assay.
One nM [3H]-CP 55,940 (126 Ci/mmol; Perkin Elmer Life Sciences, Inc) and 50 g of
membrane proteins were loaded in triplicate in a 96-well plate with increasing concentrations
of unlabelled competitive inhibitor, WIN. Dilutions of WIN were prepared to yield final
concentrations ranging from 100 fM to 10M. Non-specific binding was estimated in the
presence of 100 M WIN (Thomas et al., 1998). Samples were incubated for 70 minutes at
room temperature in a final volume of 200 μL. This period of incubation was sufficient to
ensure that equilibrium binding had been reached (Hillard et al., 1995).
A Unifilter GF/B glass fibre filter plate (Perkin Elmer Life Sciences, Inc., USA) was
pre-soaked in 40 μl of 0.1% polyethyleneimine (PEI; Sigma). The binding reaction was
terminated by rapid vacuum filtration onto the filter plate using a Unifilter Cell Harvestor
(Packard Instruments Inc.). The filter plate was washed 3 times with ice-cold 50 mM Tris
buffer, pH 7.4, containing 0.1% (w/v) BSA (washing buffer) to remove unbound [3H]-CP
55,940 and excess WIN. An important step was the rapid separation of bound radioligand
from free radioligand, which was achieved by reducing the buffer temperature in order to
slow the rate of dissociation. The filter plate was then dried at room temperature overnight.
On the following day, 40 μl of scintillation fluid (MicroScint 20, Perkin Elmer Life Sciences,
Inc., USA) was added to each well. The plate was then sealed and read by scintillation
spectroscopy (Packard TopCount, Packard Instruments Inc.). For each concentration, the non-
specific binding value was subtracted from total binding values to yield the specific binding.

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 104
4.2.2.5 Data Acquisition and Statistical Analysis
It has been shown that radioligand binding assays with [3H]-CP 55,940 have a Hill
coefficient of approximately 1 indicating a single binding site without any co-operativity
(Devane et al., 1988; Compton et al., 1993; Rodriguez de Fonseca et al., 1994; Kearn et al.,
1999; Vinod et al., 2005). Therefore, the data were fitted with a one site non-linear regression
to determine the IC50 value of each sample (Prism 5). Two-way ANOVAs followed by
Bonferroni post hoc comparisons were performed to determine whether there were
statistically significant differences between the IC50 values for the sham and BVD animals,
and between post-operative time points (SPSS 17). The data were tested for normality and
homogeneity of variance and were natural log (ln) transformed where necessary.

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 105
4.3 Results
4.3.1 Western Blotting
4.3.1.1 Optimum Sample Amount
The CB1 receptor protein band intensity was proportional to the amount of protein
loaded into each well. Twelve g of protein was chosen as an optimum sample amount, as it
gave clear bands. The integrity of the band decreased as the amount of sample loaded
increased (Figure 4.1).
Figure 4.1
(a) The CB1 receptor band intensity increased as the amount of sample protein increased. This
change can be visualised in the western blots (b) for the CB1 receptor and its corresponding
actin.
CB1
Actin
6 12 18 24 30 36 42 48
Amount of protein (g)
a)
b)

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 106
4.3.1.2 CB1 Receptor Antibody Specificity
The specificity of the CB1 receptor antibody used in the present study (Santa Cruz
Biotechnology, Inc) was validated by using the blocking peptide. The CB1 receptor specific
bands (54 and 63 kDa) disappeared when blotted with a „neutralised‟ antibody (Figure 4.2).
Although the application of the blocking peptide led to the disappearance of some bands
specific to CB1 receptor, several other bands still remained on the blot after the treatment,
which raised a concern regarding the specificity of the CB1 receptor antibody. Based on these
results, non-specific CB1 receptor labelling would be expected using these antibodies in
immunochemistry (Appendices 1 and 2).
Figure 4.2
Western blot images showing the concerning lack of specificity of the CB1 receptor antibody
for CB1 receptors. Although the CB1 specific bands at 63 and 54 kDa (arrows) are absent in the
blot performed with the blocking peptide (BP) compared to the antibody alone, the other bands
remain.
4.3.1.3 Changes in CB1 Receptor Protein Density
The CB1 receptor protein level was significantly lower in the BVD animals compared to
sham animals, only in the CA3 area across the 3 time points (F(1,33) = 4.05, P = 0.027; Figure
4.3b), with no significant interaction between surgical group and time. However, post hoc
comparisons were significant only for BVD at 1 day and sham at 7 days (P = 0.02), and BVD
at 7 days and sham at 1 day (P = 0.0001) and 3 days (P = 0.006). The expression levels of the
CB1 receptor protein were significantly reduced in all regions of the hippocampus in a time-
dependent manner (CA1, F(2,33) = 104.25, P = 0.000; CA3, F(2,33) = 20.31, P = 0.000; DG,
F(2,33) = 324.27, P = 0.000; Figure 4.3a-c, respectively). Post hoc comparisons of the CA1 and
DG areas indicated significant differences between all time points, where 1 day post-operation
had the highest and 7 days post-operation had the lowest level of the CB1 receptor protein (P
= 0.000). In the CA3 region, the level of CB1 receptor protein was significantly lower in the 7
days post-operation group compared to the 1 and 3 day post-operation groups (P = 0.000 and
P = 0.001, respectively). However, there was no significant difference between 1 and 3 days
post-operation (P = 0.100). There were no significant interactions for any of the regions.
CB1 CB1 BP
75 kDa
50 kDa

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 107
Figure 4.3
The levels of CB1 receptor protein in the (a) CA1, (b) CA3, (c) DG of the hippocampus at 1, 3,
and 7 days following BVD compared to the sham controls. Data are represented as mean +
SEM. (d) A representative blot for the CB1 receptor protein (54 and 63 kDa, bottom and top
bands, respectively) and its corresponding actin in BVD (B) and sham (S) animals for CA1 at 7
days post-operation (n = 6 for sham and n = 7 for BVD animals).
CB1
Actin
`B S `B S B S `B S B S B S B
a) b)
c) d)

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 108
4.3.2 Radioligand Binding Assay
4.3.2.1 Optimisation of the Protein Amount and Radioligand Concentration
The specific binding of [3H]-CP 55,940 was higher in the wells containing 1 nM of
[3H]-CP 55,940 compared to 0.5 nM (Figure 4.4a). A 1 nM concentration was chosen because
the higher concentration of [3H]-CP 55,940 gave higher radioactivity, therefore any change in
the binding may be detected with greater sensitivity than with a lower concentration. Figure
4.4b shows that 100 L of WIN in non-specific binding wells was sufficient to inhibit [3H]-CP
55,940 binding. Although the total binding increased with the increase of the protein amount
in the well, the level of non-specific binding remained relatively unchanged. This was
demonstrated by a low R2 value for the non-linear regression analysis, in which the line of
best fit for the non-specific binding was almost horizontal (R2 = 0.872 for total binding and
0.160 for non-specific binding; Figure 4.4b).
Figure 4.4
(a) Saturation binding curve with increasing amount of hippocampal membrane preparation for
different concentrations of [3H]-CP 55,940. (b) Specific binding of [
3H]-CP 55,940 was calculated
from the difference between total and non-specific binding. Data are represented as mean
counts per minute (CPM) + SEM.
a)
b)

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 109
4.3.2.2 Changes in the CB1 Receptor Binding Affinity
There were no significant differences in the CB1 receptor binding affinities between the
sham and BVD animals at any post-operative time points, and there were no significant
interactions (Figure 4.5).
Figure 4.5
Displacement curves of the [3H]-CP 55,940 binding with WIN as a competitive inhibitor and the
IC50 values of WIN at (a, b) 1 day, (c, d) 3 days, (e, f) 7 days following sham or BVD surgery.
Data are represented as mean + SEM.
a) b)
c) d)
e) f)

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 110
4.4 Discussion
In the present study, changes in the density and affinity of CB1 receptors in the
hippocampus were investigated at specific time points following BVD. This was the first
study to show that BVD results in significant changes in the expression of the CB1 receptor in
a specific sub-region of the hippocampus. The density of CB1 receptor expression was
significantly lower in the BVD animals than in the sham animals in the CA3 region of the
hippocampus, but in no other hippocampal region investigated. Similar to the CB1 receptor
density decrease following BVD, a decrease in the level of CB1 receptors in the hippocampus
is generally observed following chronic administration of exogenous cannabinoids, as part of
the development of the animals‟ tolerance to the cannabinoid drugs (Romero et al., 1998;
Rubino et al., 2000a, b; Hill et al., 2004; Dalton et al., 2009) or in response to chronic stress
(Hill et al., 2005, 2008b, 2009; Reich et al., 2009; Suàrez et al., 2009). Furthermore, down-
regulation of CB1 receptor expression in other parts of the brain such as the basal ganglia and
the striatum can be observed in certain pathological conditions such as Huntington‟s disease
(Richfield and Herkenham, 1994; Glass et al., 1993, 2000; Allen et al., 2009; Dowie et al.,
2009; see Pazos et al., 2008 for review) and multiple sclerosis (Berrendero et al., 2001;
Cabranes et al., 2006; Cetonze et al., 2007; see Centonze et al., 2008 for review).
There are several possible explanations for the observed region-specific reduction of
CB1 receptor density in the BVD animals. First, it has recently been shown that the CA3
pyramidal neurons dynamically control their inhibitory inputs through the Group I
metabotropic glutamate receptor-mediated release of endocannabinoids (Inada et al., 2010).
Furthermore, Kim and Alger (2010) demonstrated the homeostatic mechanism of
endocannabinoid signaling in activity-deprived CA1 pyramidal neurons, where the afferent
excitation of the CA1 region was reduced by the surgical removal of the CA3 area. It has been
shown that neuronal networks are maintained by homeostatic compensation of synaptic
strength when long-lasting alterations in neuronal activity occur (see Turrigiano, 2008 for
review). Since studies have shown that the endocannabinoid system has a neuromodulatory
function in the hippocampus (see Section 1.2.2 of Chapter 1; Breivogel and Sim-Selley, 2009
for review) and that BVD results in severe hippocampal place cell dysfunction (Stackman et
al., 2002; Russell et al., 2003b, 2006), it can be hypothesised that decreased CB1 receptor
density in the CA3 region in BVD animals might be a part of a homeostatic mechanism for
changes neuronal activity in the hippocampus following BVD.

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 111
Second, previous studies have suggested that the CA3 region of the hippocampus is
important in memory formation (Hess et al., 1995; Gall et al., 1998; Zhao et al., 2000; He et
al., 2002). Since the endocannabinoid system is known to have an important role in learning
and memory (see Davies et al., 2002; Lichtman et al., 2002 for reviews) and many studies
have shown that BVD animals are cognitively impaired in a number of learning and memory
tasks (results from Chapter 3; Wallace et al., 2002c, Zheng et al., 2004, 2007, 2009b), this
decrease in CB1 receptor density in the CA3 region in BVD animals might be an initial
pathological indication of the functional deficits such as spatial memory impairment that are
associated with BVD. However, further studies will be required to determine the functional
significance of the changes in CB1 receptor expression in the CA3 region of the hippocampus
following BVD that were found in the present study.
Third, the decreased CB1 receptor density observed in the CA3 region in the current
study might be associated with degeneration in specific hippocampal structures, which might
include changes in synaptic proteins, dendrites, or even hippocampal cell death. Changes in
proteins that are related to synaptic transmission and neuronal plasticity in the hippocampus
have already been documented in BVD rats. These proteins are synaptophysin, synaptosomal-
associated protein 25 (SNAP-25), neurofilament light subunit (NF-L), and drebrin (Goddard
et al., 2008c). However, it has been observed that there is a significant increase in SNAP-25
only in the DG region of the hippocampus in BVD animals compared with sham animals,
with no further changes in other proteins (Goddard et al., 2008c). Although widespread
apoptosis has been observed in the hippocampus following BVD in rats (Smith et al., 2009),
this was a preliminary observation, and therefore more systematic quantification is required to
further clarify whether the decrease in CB1 receptor density found in the present investigation
is due to a decrease in cell number or instead due to a decrease in the number of CB1 receptors
expressed per cell.
In the present study, the density of CB1 receptor expression decreased in a time-
dependent manner in all sub-regions of the hippocampus following both the sham and BVD
surgeries. It was particularly interesting to note that even the sham surgery resulted in a
decrease in CB1 receptor density over time, to the same extent as for the BVD animals. An
age-related decrease in the density of hippocampal CB1 receptors has been reported in rats
(Berrendero et al., 1998; Marchalant et al., 2008; Canas et al., 2009) and in humans (Mato
and Pazos, 2004; Wong et al., 2010). However, in these studies, the effect of age on CB1
receptor density was investigated over a long period of time, which ranged from 2-24 months

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 112
for rats and 21-73 years for humans. The time-dependent decrease in CB1 receptor density
observed in the present study is unlikely to be due to aging because, not only were the animals
3 months old at the time of tissue collection, the particular time points used in the present
study (1, 3 and 7 days post-operation), are relatively short-term time points compared to those
of the above aging studies (Berrendero et al., 1998; Mato and Pazos, 2004; Marchalant et al.,
2008; Canas et al., 2009; Wong et al., 2010). For these reasons, future similar studies should
include a separate non-surgical control group for every time point to clarify whether the sham
surgery has any effect on the density of the CB1 receptors in the hippocampus.
Despite the number of studies showing a reduction in CB1 receptor density following
chronic stress or due to tolerance, studies investigating temporal changes in CB1 receptor
levels are scarce. A time-dependent decrease in CB1 receptor expression has been reported in
animal models of epilepsy (Falenski et al., 2009) and Parkinson‟s disease (Walsh et al., 2010),
with similar post-operative time points to the present study (4 and 7 days for Falenski et al.,
2009; 1, 3, 7, 14, 28 days for Walsh et al., 2010). This suggests the possibility that the
endocannabinoid system is highly sensitive to perturbations of any sort, and that even sham
surgery causes a transient up-regulation of CB1 receptor expression that gradually decreases
over the course of one week. Furthermore, it is possible that these temporal changes in CB1
receptor expression are not restricted to the hippocampus alone. Recently, it has been
hypothesised that effective performance in working memory tasks may involve prefrontal
cortical functionality in addition to the important role of the hippocampus. Furthermore, not
only are CB1 receptors expressed at a high level in the prefrontal cortex, it has been shown
that prefrontal cortex activity is altered by the administration of cannabinoids (see Egerton et
al., 2006 for review). For these reasons, the prefrontal cortex would be one of many areas to
target in future investigations into whether the expression of the CB1 receptor changes with
time following BVD.
The second step in determining whether BVD results in changes to CB1 receptors, was
to investigate possible changes in CB1 receptor binding affinity, using the [3H]-CP 55,940
binding assay. While there was no significant difference in CB1 receptor affinity between the
sham and BVD rats, it was interesting to find that the IC50 values obtained from the present
study varied between 16 – 43 nM. Astonishingly, no previous study has assessed the IC50 of
WIN in the hippocampal membrane preparation. Nonetheless, Houston and Howlett (1998)
reported an IC50 for WIN of 38 nM in a [3H]-CP-55,940 competitive binding assay using the
whole brain membrane preparation. Although Thomas et al. (1998) did not report an IC50 for

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 113
WIN, this value could be calculated from the data given, by using the Cheng-Prusoff equation
(Cheng and Prusoff, 1973), revealing an IC50 value of 27 nM, but again in the whole brain
membrane preparation. Using a single neuron or primary neuron culture, 47 and 14 nM were
reported as IC50 values for WIN, respectively (Pan et al., 1996; Shen and Thayer, 1998).
Taken together, although the IC50 values in the present study are within the range of results
from previous studies, a direct comparison cannot be made because the present study used a
hippocampal membrane preparation rather than a whole brain preparation. For this reason, a
non-surgical group should be included in future studies to determine whether the sham
surgery has any effect on CB1 receptor affinity.
Although there was no significant difference in the binding affinity of the hippocampal
CB1 receptors between the sham and BVD animals, the present study is the first study to
report the IC50 for WIN, and hence the binding affinity of the CB1 receptors for WIN, in the
rat hippocampus. In addition to a scarcity of literature on the IC50 for WIN in the brain, there
is also no study to date reporting the Ki for WIN in the hippocampus. However, there are two
studies that used the whole brain membrane preparation (Thomas et al., 1998; Gullapalli et al.,
2010). This was surprising as the endocannabinoid system and its involvement in many
pathological conditions has been studied thoroughly, yielding countless publications.
However, the majority of these studies have focused on the density or the efficacy of the
cannabinoid receptor, and only a very few studies have investigated the binding affinity.
In the present study, a competitive binding assay was performed, in which the
concentration of the [3H]-CP 55,940 remained constant (1 nM), while the concentration of the
unlabelled ligand (WIN) was varied. The binding affinity of a receptor can be expressed in
various ways, including measurements such as Kd, Ki, and IC50. The IC50 in the present study
was estimated instead of Ki, as the Prism 5 software required the use of limiting constraints
when performing the analysis to estimate Ki. These constraints were the concentration of the
radioligand used in the binding assay and more importantly, the Kd of the radioligand.
Without entering the values for these constraints, the programme would not allow non-linear
regression to fit a model to directly estimate Ki. Although it was simple to perform this
analysis, changing these constraints inevitably changed the regression, therefore, any estimate
from the line of best fit (that is, values for Ki) also changed. Therefore, it would have been
necessary to choose these constraints very cautiously and with considerable confidence in
their accuracy. The concentration of the [3H]-CP 55,940 used in the present study was 1 nM.
As a saturation binding assay was not performed in the current study, the Kd of the [3H]-CP

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 114
55,940 had to be obtained from the literature. However, the reported Kd of the [3H]-CP 55,940
in the hippocampus varied between 0.5 – 1.7 nM (Romero et al., 1995; Gatley et al., 1997;
Castelli et al., 2007; Hill et al., 2005, 2008a, 2010), which consequently resulted in a large
variation in Ki estimation. For this reason, it was necessary to consider approaches other than
Ki to estimate the affinity of the CB1 receptor.
Fortunately, the non-linear regression analysis to estimate IC50 did not require the use of
any limiting constraints. Since Ki is proportional to IC50 (Motulsky, 1995), the IC50 for WIN
could also be used to quantify the binding affinity of the CB1 receptors in the hippocampus.
However, a disadvantage of estimating the IC50 is that by changing the experimental
conditions, such as changing the radioligand used or changing its concentration, the IC50
would also change (Motulsky, 1995). In other words, increasing the radioligand concentration
used or using a radioligand with a lower Kd (that is, a ligand with higher affinity) would
increase the IC50, as a larger concentration of unlabelled ligand would be required to compete
for the binding site when the radioligand concentration is high, or more unlabelled ligand
would be required to compete for a high affinity radioligand (low Kd) than for a low affinity
radioligand (high Kd).
Ideally, a saturation binding assay with the [3H]-CP 55,940 should have been performed
in order to obtain the CB1 receptor affinity more directly. However, due to a greater amount of
radioligand required to perform the saturation binding assay compared to the competitive
binding assay, it would have been prohibitively expensive to run 45 separate saturation
binding assays for the present study. Nevertheless, another option might have been to perform
the saturation binding assays for a small number of the samples to obtain the Kd of the [3H]-
CP-55,940, and then to carry out the competitive binding assays to obtain the Ki. Such
possibilities should be considered in future studies of a similar nature.
Numerous studies have shown the distribution and localisation of the CB1 receptors in
the brain (Pettit et al., 1998; Tsou et al., 1998; Ong and Mackie, 1999; Moldrich and Wenger,
2000; Egertová and Elphick, 2000; Suárez et al., 2008, 2009). In order to provide a complete
picture of effects of BVD on CB1 hippocampal receptors, the expression of hippocampal CB1
receptors following BVD was also examined in the present study by means of
immunohistochemistry (Appendix 2), using the same CB1 receptor antibody that was used for
western blotting in this Chapter. However, since multiple protein bands were observed with
this CB1 receptor „specific‟ antibody in western blotting, it is possible that these other bands

Chapter 4: Changes in CB1 Receptor Density & Affinity Following BVD 115
that are recognised by the CB1 antibody may similarly produce non-specific CB1 receptor
labelling in immunohistochemistry. The lack of the specificity of the CB1 receptor antibody
has also been observed with other commercial CB1 receptor antibodies (Sigma, Affinity
BioReagents, Cayman, and BoiSource International; Grimsey et al., 2008; Jelsing et al.,
2008). Although the use of tissues from the CB1 receptor knockout mice would have been the
easiest way to clarify the specificity of the CB1 receptor antibody, such tissues were not
available for the present study. Taken together, although the specificity of the CB1 receptor
antibody was tested by varying antibody concentrations and incubation durations (Appendix
1), due to considerable doubt regarding the binding specificity of the CB1 receptor antibody,
the labelling obtained in immunohistochemical study is considered questionable (Appendix 2).
The particular time points that were chosen for the present study (1, 3 and 7 days post-
operation) were relatively short post-operation time points compared to a 14 months post-
operation time point in the foraging task (Chapter 3), and therefore it is possible that
behavioural results from Chapter 3 are not directly comparable with the results presented in
the present Chapter. Furthermore, because these are acute time points, the changes observed
in CB1 receptor density and affinity in the present study might be related to the immediate
effects of the loss of vestibular function rather than to its long-term consequences. However, it
has been shown that hippocampal place cell function is disrupted immediately following BVD
(1 hr post-BVD induction, Stackman et al., 2002), and there is no evidence of recovery from
BVD-induced spatial memory deficits (see Chapter 3; Zheng et al., 2009b). Thus, it was
logical to analyse CB1 receptor density and affinity at acute time points following BVD,
rather than at long-term time points. Nevertheless, since CB1 receptor density and affinity did
not change substantially at 1, 3 and 7 days post-operation, longer post-operative time intervals
should be analysed in future studies to determine whether there are long-term changes in the
endocannabinoid system following BVD.

116
Chapter 5
Discussion and Conclusions

Chapter 5: Discussion and Conclusions 117
There has been an accumulation of evidence suggesting that damage to the peripheral
vestibular system results in the disruption of hippocampal synaptic transmission and cognitive
function. Over the past several years, it has been firmly established that the endocannabinoid
system is of fundamental importance in hippocampal synaptic plasticity and cognitive
function. The key aim of this thesis was to determine the involvement of the endocannabinoid
system in the cognitive deficits caused by bilateral loss of vestibular function, in order to
elucidate the potential mechanism(s) that may underlie this impairment. The results from the
present study suggest that cognitive deficits following BVD may be permanent and provide
some preliminary evidence that the role of the hippocampal endocannabinoid system in the
BVD-induced cognitive deficits may be limited.
Previous studies involving rats with BVD have demonstrated spatial memory deficits
that appear to be long-lasting, suggesting long-term adverse effects on the hippocampus
(Russell et al., 2003b; Zheng et al., 2009b). However, the longest post-operative time interval
that had been studied before the present investigation was approximately 5-7 months post-
surgery (Zheng et al., 2009b). The results from the present study suggest that rats with BVD
probably do not recover from spatial memory deficits even at very long time intervals
following the surgery, such as 14 months (Chapter 3), suggesting that deficits enduring at this
long time point may well be permanent. These apparently permanent learning and memory
impairments following BVD are of significance as approximately 80% of patients with
bilateral vestibulopathy do not recover significantly (Zingler et al., 2008) and ongoing
memory problems may contribute to this phenomenon. Although the effects of cannabinoid
drugs on BVD animals‟ spatial memory were more complicated than expected, some
speculation could be made based on the observed changes in CB1 receptor density and affinity
following BVD (Chapter 4).
Although a two-way ANOVA showed that BVD significantly decreased CB1 receptor
density in the CA3 region of the hippocampus across 3 post-operation time points – 1, 3 and 7
days, post hoc tests revealed that none of the comparisons for the same individual post-
operation time points were significant (Chapter 4). Therefore, although there was evidence for
a decrease in CB1 receptor density in BVD animals, it was not as convincing as it might have
been. The observed down-regulation of CB1 receptor density following BVD may reflect
either a decrease in synthesis rate or an increase in degradation rate of receptors. One possible
explanation for the down-regulation of CB1 receptors in CA3 is that this reduction is due to a
homeostatic response to increased levels of endocannabinoids in the hippocampus. Not only

Chapter 5: Discussion and Conclusions 118
has the enhancement of endocannabinoid levels been observed following various pathogenic
insults in the brain (Panikashvili et al., 2001; Marsicano et al., 2003; Wallace et al., 2003; de
Lago et al., 2006; van der Stelt et al., 2006), endocannabinoids have been shown to provide
protection against these neuronal insults (see Fowler et al., 2010 for review).
There is increasing evidence that the integrity of the hippocampus relies on input from
the vestibular system. The impaired performance of BVD animals in the foraging task
(Chapter 3), together with results from previous studies (Stackman and Herbert, 2002; Russell
et al., 2003a; Zheng et al., 2006, 2007, 2009b), have shown that BVD results in impairment in
various spatial memory tasks that are known to be sensitive to disruption of hippocampal
functions in animals. In addition, neurophysiological studies have shown that BVD causes
abnormalities in the function of place cells and in theta rhythm in the hippocampus (Stackman
et al., 2002; Russell et al., 2003b, 2006). In humans, bilateral hippocampal atrophy has been
observed following BVD (Brandt et al., 2005), while in animals, BVD appears to induce
apoptosis in the hippocampus (Smith et al., 2009). Taken together, it is evident that BVD
adversely affects the hippocampus, and therefore, it is conceivable that endocannabinoid
levels in the hippocampus might be elevated following BVD as observed in other
neuropathological conditions. For this reason, it will be interesting in future studies to
determine whether the level of endocannabinoids in the hippocampus changes following BVD.
The consequence(s) of decreased CB1 receptor density in the CA3 region of the
hippocampus in BVD animals (Chapter 4) and whether this decrease might be relevant to
BVD-induced cognitive deficits, remains to be elucidated. Why the CB1 receptor should be
affected only in the CA3 region of the hippocampus is also unclear at present. It is possible
that the reduction in CB1 receptor expression in CA3 following BVD might be related either
to the adverse effects of vestibular loss on the hippocampus, or to compensatory processes
initiated in response to the injury. However, despite the significant difference from the sham
controls, the possibility that any sensory loss might have caused similar changes, must be
considered.
Numerous pharmacological and brain lesion studies have shown that the CA3 region of
the hippocampus is important for spatial information processing (Routtenberg, 1984; Stubley-
Weatherly et al., 1996; Roozendaal et al., 2001; Steffenach et al., 2002; Stupien et al., 2003;
Kesner, 2007; Holahan and Routtenberg, 2010). Similar to the heading angle measurements in
the foraging task of the present study (Chapter 3), Holahan and Routtenberg (2010) also

Chapter 5: Discussion and Conclusions 119
measured rats‟ heading angles towards a hidden platform from the starting point in the water
maze task, where the ideal heading was a straight line from the launch point to the platform.
In their study, lidocaine was injected directly into the CA3 region of the hippocampus, which
locally inactivated the neural activity in the granule cell-mossy fibre CA3 circuit. Interestingly,
like BVD animals in the present study, animals treated with lidocaine showed a significant
deviation from the ideal heading angle in the water maze task (Holahan and Routtenberg,
2010). These results suggest that the CA3 region of the hippocampus may be important for
processing vestibular information, which in turn could contribute to the cognitive impairments
induced by BVD. This hypothesis leads to a potential future investigation involving
intrahippocampal administration of cannabinoids to BVD animals, which would provide
direct evidence of whether the cannabinoid receptors in the hippocampus are involved in
BVD-induced cognitive deficits.
It is currently unknown which specific regions of the hippocampus receive vestibular
information first. However, it may be reasonably supposed that a decrease in CB1 receptors in
the CA3 region is likely to interfere with CA3 output to CA1 via the Schaffer collateral
pathway. It has been shown that the hippocampal input pathway from the DG to the CA1
region is unidirectional. Although the granule cells in the DG project to the pyramidal cells in
the CA3 region via mossy fibre projections, pyramidal cells of CA3 do not project back to the
granule cells. Likewise, the pyramidal cells of the CA3 region project to CA1 via Schaffer
collaterals, but CA1 does not project back to CA3 (Amaral and Lavenex, 2007). Since the
CB1 receptors are known for modulating synaptic transmission by regulating the release of
major neurotransmitters such as glutamate and GABA (see Turu and Hunyady, 2010 for
review), it is possible that a decrease of the CB1 receptors in the CA3 region, as seen in the
BVD rats, results in a decrease in the release of glutamate and GABA, which in turn reduces
synaptic transmission to CA1. Thus, a reduction in CB1 receptor density in the CA3 region of
the hippocampus in BVD animals might be a part of the initial stages of the pathogenesis
which contributes to the emergence of cognitive deficits following BVD. Given that the
responsiveness of place cells in the CA1 region is severely disrupted following BVD
(Stackman et al., 2002; Russell et al., 2003b), it is conceivable that the down-regulation of
CB1 receptors in the CA3 region may contribute to the impairment of place cell function and
spatial memory following vestibular lesions.
Ashton et al. (2004b) showed that there were no significant changes in CB1 receptor
density in the hippocampus at 10 hours and 2 weeks following UVD. However, given that

Chapter 5: Discussion and Conclusions 120
UVD causes an imbalance in activity between the two brainstem vestibular nuclei (Smith and
Curthoys, 1989), the effects of UVD on the endocannabinoid system in the hippocampus are
likely to be different compared to BVD. Therefore, further studies will be required to
determine the functional significance of the changes in CB1 receptor expression in the CA3
region of the hippocampus following BVD that were found in the present study (Chapter 4).
As the method of western blotting was used to determine the density of CB1 receptors in
the hippocampus, it is not possible to know whether this reduction occurred in neurons or
glial cells, or both. In the present study, immunohistochemistry was carried out to further
examine the change in CB1 receptor expression following BVD (Appendix 2). However, one
of the major shortcomings of the present study was the lack of specificity of the CB1 and CB2
receptor antibodies (Chapter 4; Appendix 3). Although the optimum protocols for labelling
CB1 (Appendix 1) and CB2 (Appendix 3) receptors were determined, due to the lack of
specificity of the commercially available CB1 and CB2 receptor antibodies (Chapter 4;
Appendix 3; Grimsey et al., 2008; Jelsing et al., 2008), concerns were raised regarding the
reliability of the immunohistochemical data obtained in the present study to determine
changes in CB1 receptor density (Appendix 2).
To completely characterise changes in CB1 receptors following BVD and their
involvement in BVD-related cognitive deficits, further studies will be required to determine
the functional significance of these changes (Chapter 4). It is possible that they are related to
the impairment of place cell function and theta rhythm following BVD. It is also conceivable
that changes take place in the G-protein coupling of the CB1 receptor. Possible experiments to
investigate these possibilities include using a [35
S]GTPS binding assay to measure the level
of G-protein activation upon CB1 receptor stimulation, and some biochemical assays which
measure the level of cAMP and protein kinase A activity. The latter assays are logical as the
cAMP cascade is one of the major intracellular signaling pathways implicated in the
biological actions of cannabinoids. As well as investigating the CB1 receptor and its related
protein expression, CB1 receptor mRNA expression should also be studied using in situ
hybridization to determine whether the BVD changes the genetic expression of CB1 receptors
in the hippocampus. Furthermore, other components that comprise the endocannabinoid
system, that is, the endocannabinoids and the enzymes that break down endocannabinoids,
could also be assessed. Knowledge of the changes in the level of endocannabinoids and the
activity of FAAH and MAGL (enzymes responsible for the breakdown of endocannabinoids)
in the brain following BVD, would further indicate whether the endocannabinoid system is

Chapter 5: Discussion and Conclusions 121
involved in the functional changes in the hippocampus that occur following BVD.
Many believe that the effects of cannabinoids are influenced by the expression of CB1
receptors in the brain (Herkenham et al., 1991, Jansen et al., 1992; Sim et al., 1995), but the
possibility of changes in the affinity of these receptors are often neglected. It was interesting
to observe that while there was a change in receptor expression following BVD, there was no
change in the affinity of the CB1 receptors for WIN, as IC50 values for WIN were not different
between the sham and BVD animals (Chapter 4). Similar results were found by Hill et al.
(2005, 2008a), in which both chronic stress and repeated administration of corticosterone in
rats significantly decreased hippocampal CB1 receptor density without affecting affinity for
the CB1 receptor agonist, [3H]-CP 55,940. The results from the present study, together with
the Hill et al. studies, suggest that one cannot assume that the changes in receptor expression
would result in changes in affinity.
Much of our understanding of vestibular-hippocampal connections comes from clinical
and experimental observations in humans and animals with unilateral and bilateral vestibular
lesions. However, there have been some studies suggesting that the stimulation of the
vestibular system either by cold water (i.e. caloric stimulation; Bachtold et al., 2001) or by
low intensity electrical stimulation (i.e. galvanic stimulation; Wilkinson et al., 2008, 2010)
may improve some aspects of memory in humans, although this has not been investigated for
spatial memory as yet (see Smith et al., 2010 in press for review). Similarly, McGauran et al.
(2005) showed that vestibular stimulation by rotation, enhanced rats‟ performance in the water
maze task. Since the present study investigated the effects of vestibular lesions on the
endocannabinoid system in the brain, it would be interesting to also investigate whether or not
the endocannabinoid system is affected by vestibular stimulation.
Clearly, the mechanisms contributing to the effects of BVD on cognitive functioning
require further investigation. Nevertheless, the results from the foraging task have confirmed
that inputs from the vestibular system are crucial for spatial navigation and memory in
animals. The BVD animals were unable to use not only self-movement cues, but also
allothetic cues in navigation (Chapter 3). This result is consistent with the results of Brandt et
al. (2005) in humans, and suggests that spatial memory deficits in patients with vestibular
dysfunction may become worse over time.
In the present study, although it was expected that a cannabinoid receptor agonist would

Chapter 5: Discussion and Conclusions 122
impair spatial memory (Suenaga and Ichitani, 2008; Robbe and Buzsaki, 2009; Wise et al.,
2009), WIN did not seem to impair spatial memory even in the sham animals, as would be
expected (Chapter 3). In fact, WIN at 2 mg/kg, significantly improved BVD animals‟
performance in the dark, but not in sham animals, suggesting that BVD might have resulted in
changes in the brain‟s endocannabinoid system. Furthermore, the pre-treatment with the
cannabinoid receptor inverse agonist, AM251, demonstrated that the involvement of the
endocannabinoid system in the BVD-induced cognitive deficits is complicated.
In conclusion, evidence presented in this thesis indicates that bilateral vestibular loss
may cause permanent spatial memory deficits in animals, and lays a foundation for further
investigation of the involvement of the endocannabinoid system in the debilitating effects of
vestibular damage.

123
Appendices

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 124
Appendix 1
The Specificity of Cannabinoid CB1 Receptor Labelling in
the Rat Hippocampus
1.1 Introduction
In order to reliably compare the effects of surgery or drug treatment on the expression of
CB1 receptors in the brain, it is essential to have sufficient controls to convince a sceptical
observer that the staining patterns obtained using antibodies in the present study, really do
represent the CB1 receptor. Confirming the specificity of CB1 receptor antibodies was
therefore the first step necessary before investigating the effects of vestibular damage and
cannabinoid treatment in the rat.
A considerable amount of work has been done to clarify the molecular mechanisms
responsible for the effects of cannabinoids on cell function and behaviour in animals. Since
the discovery of the CB1 (Devane et al., 1988) and CB2 receptors (Munro et al., 1993), the
focus of cannabinoid research has been on the molecular events that alter the behaviour of
cannabinoid receptors. An important issue that needs to be dealt with before studying CB1
receptor function is to confirm where and how these receptors are expressed and distributed.
Many rat studies have been published on the distribution and localisation of the CB1 and CB2

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 125
receptors in the brain and in the immune system, respectively. Numerous conventional
methods such as receptor autoradiography, in situ hybridisation, and immunohistochemistry
have been utilised to clarify the distribution and the localisation of these two receptors in the
rat brain. The common principle behind these methods is the formation of the receptor-ligand
complex. Here, the ligand can be a radiolabelled agonist or antagonist, a complementary DNA
(deoxyribonucleic acid) or RNA (ribonucleic acid) probe, or a specific antibody that
recognises a particular protein, the CB1 receptor in this case. Antibodies are large protein
molecules that are able to identify an epitope found on a protein. The epitope may be in a
certain molecular configuration in the native protein, or it can be exposed in the fixed
molecule that is not present in the native configuration (see Saper and Sawchenko, 2003 for
review). When the antibody recognises an epitope, it binds to that specific epitope and the
bound antibody can be visualised in various ways by using an enzyme-conjugated secondary
antibody that catalyses a colour-producing reaction or using a secondary antibody that has
been labelled with a fluorophore.
A crucial issue in studying endogenous cannabinoid receptor proteins in the brain and in
other areas, is the availability and specificity of the antibodies used. For the past decade, CB1
receptor antibodies, targeting different regions of the receptor, have become indispensable
research tools and have been manufactured by numerous research groups around the world. A
diverse range of CB1 antibodies can also be purchased through a variety of commercial
suppliers. However, concerns have been raised regarding the specificity of the antibodies in
labelling CB1 receptors, since it is rare that an antibody is exclusively specific to the desired
target protein. Due to this unreliable antibody specificity, immunohistochemistry has
sometimes yielded variable and even flawed results with various antibody preparations. It is
important to realise that the antibodies used in immunohistochemistry are biological agents,
not ordinary chemical reagents. Therefore, it is highly likely that antibodies may recognise a
broad range of antigens, rather than only the antigen they were raised to recognise, and thus
one should always consider that specific antibody labelling is limited and that even the most
specific antibody available may label not only the actual antigen, but also other “antigen-like”
molecules.
In order to minimise misrepresentation of antigens, many investigators have chosen to
produce their own antibodies rather than to buy them from commercial companies. However,
unsatisfactory immunohistochemical results do not solely depend on whether the antibody
was made privately or commercially bought. There are many other possible factors that may

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 126
affect the end results. These include preparation of the tissue, tissue thickness, the host of the
antibody, antibody incubation time, the antibody‟s binding site, whether the antibody binds to
the N-terminal or C-terminal domains of the receptor, and the antibody concentration (Lorincz
and Nusser, 2008; see Goldstein et al., 2007 for review). Considering each of the above
factors, it is clear that a series of steps must be taken to control for these variables. Previous
immunohistochemical studies have shown surprising discrepancies in the specificity of CB1
receptor antibodies, in which the results obtained were contradictory to one another.
Furthermore, a standard method for carrying out immunohistochemistry to label CB1
receptors in the brain was absent in these studies. In order to control for this inconsistency, at
least to some extent, Grimsey and others have recently tested the ability of a series of CB1
antibodies to detect endogenous CB1 receptors in cultured cells and in brain slices (Grimsey et
al., 2008). In this study, four commercially available (Sigma, Affinity BioReagents, Cayman,
and BoiSource International) and two privately made CB1 antibodies were examined.
Theoretically, all CB1 receptor antibodies should recognise the same CB1 receptor antigen,
and hence give a similar labelling pattern. However, the CB1 receptor antibodies from
different providers showed notably different CB1 receptor labelling patterns (Grimsey et al.,
2008). This study raised even more doubts about the specificity of the CB1 receptor antibodies
and whether the staining patterns observed in any study truly represent the CB1 receptor.
Thus it is necessary to validate CB1 receptor antibody specificity in any study where
immunohistochemistry for CB1 receptors is employed. For this reason, it was crucial to
optimise the protocol for immunohistochemical labelling of the CB1 receptors before
investigating the effects of BVD on the expression of the CB1 receptors.

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 127
1.2 Materials and Methods
1.2.1 Removal of Brain
Three male Wistar rats, each weighing 250-300 g, were decapitated without anaesthesia.
The whole brain was rapidly removed from the skull and immediately placed into ice-cold
0.9 % saline solution. The brain was dissected on a cold plate into two parts, i.e. the forebrain
and the hindbrain. The forebrain was further divided in half in the sagittal plane. The brains
were completely covered with O.C.T. compound (BDH Laboratory Supplies), and were then
immersed in n-hexane solution (Biolab) that had been placed in liquid nitrogen. After the
O.C.T. compound had turned to white, the tissue was wrapped in aluminium foil and stored at
-20o
C until sectioning. CB1 and CB2 receptor labelling was also trialed using
paraformaldehyde-fixed tissue. However, the CB2 receptor labeling quality was very poor
when fixed tissue was used, which prohibited using the fixed tissue. In order to equalise the
tissue conditions for both CB1 and CB2 receptor labeling, fresh tissue was used for CB1
receptor labeling as well. Hydrogen peroxide was used to quench any endogenous peroxidase.
1.2.2 Tissue Sectioning
Prior to tissue sectioning, the glass slides were coated with a Poly-L-Lysine solution
(Sigma) in order to improve adherence of the frozen sections. Briefly, slides were immersed
in Poly-L-Lysine solution that had been diluted with distilled water (1:10) for 5 minutes. The
slides were then drained and dried in a drying oven at 60o C for 1 hr. Once the slides were
completely dried, they were cooled and kept at room temperature until use.
All tissue sectioning was performed at -18oC with a 16 m section thickness using a
Cryostat (Leica CM 1850). Each brain was cut in the sagittal plane using a stereological
method. Briefly, after a random start using a random number generated by a calculator, a
systematic subset of these sections separated by a fixed distance (d), was collected. Twelve
levels of the hippocampus were taken per animal where each section in each level was
separated by 464 m. Systematic random sampling was used to achieve a lower coefficient of
error compared with random sampling. The sections were thaw-mounted on Poly-L-Lysine
coated slides and stored at -20o C until staining.

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 128
1.2.3 Immunohistochemistry
Sections were air-dried briefly at room temperature to eliminate moisture that appeared
immediately after the sections were removed from -20o
C. Hydrophobic boundaries were
drawn around the sections using a DAKO pen (DAKO), and the sections were dried for a
further 5 minutes. Each step was carried out at room temperature unless otherwise stated.
When the hydrophobic ring was completely dried, sections were washed with 0.01 M
phosphate-buffered saline (PBS, Sigma), then fixed in 4% paraformaldehyde (PFA, Sigma)
for 10 minutes. To terminate PFA fixation, 0.1 M glycine (Sigma) was applied to sections for
15 minutes. Sections were treated with 0.3% H2O2 (Merck) in methanol (BDH) for 20
minutes to block endogenous peroxidase activity, and then washed with 0.01 M PBS for 5
minutes twice. In order to block non-specific binding, sections were incubated with 1.5%
heat-inactivated donkey serum (Sigma) for 1 hr. This blocking solution was prepared in 0.01
M PBS containing 0.2% bovine serum albumin (BSA, Gibco). The concentration of the
blocking solution used in the present study was utilised from the previous studies (Ashton et
al., 2004a, b; Fusco et al., 2004). After blocking was completed, various concentrations of
polyclonal CB1 receptor goat primary antibody (Santa Cruz Biotechnology, Inc.) were applied
and incubated for either 18 hrs (overnight) or 41 hrs (2 nights) at 4o C (Table 1.1). Both
primary and secondary antibody solutions were diluted in antibody-diluting buffer containing
1% BSA and 0.5% Triton X-100 (BDH) in 0.01 M PBS.
Following the primary antibody incubation, the sections were washed four times for 15
minutes each wash, with the antibody washing buffer (1% BSA and 0.5% Triton X-100 in
0.01 M PBS) to reduce non-specific binding prior to the secondary antibody application.
The sections were then incubated with an HRP-conjugated polyclonal donkey anti-goat
antibody (Santa Cruz Biotechnology, Inc.), diluted at various concentrations (Table 1.1). The
secondary antibody was either incubated for 1 or 2 hrs at room temperature. The
immunohistochemical complex was visualised by exposure to diaminobenzidine (DAB,
Sigma) for 20 minutes. Positive immunoreactivity to the CB1 receptor was expressed as
brown staining in the cytoplasm, cell body, axons, dendrites, and fibres. Negative controls
consisted of sections treated as detailed above without the primary antibodies. The stained
sections were rinsed with distilled water (dH2O) twice and then twice with tap water, for 5
minutes each time. After rinsing, the sections were dehydrated in ascending concentrations of
alcohol; 5 minutes in each of 70% and 95% ethanol, 10 minutes in 100% ethanol and 5
minutes in xylene, and cover-slipped with DPX mounting medium (a mixture of xylene and
dibutyl phthalate, BDH).

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 129
Table 1.1
Various combinations of CB1 receptor primary (1o) and subsequent secondary (2
o) antibody
titres trialled and their incubation durations.
1o antibody
concentration
2o antibody
concentration
1o / 2
o antibody incubation
time (hrs)
1:50 1:500
18 / 1
1:100 1:200
1:100 1:500
1:200 1:500
1:200 1:1000
1:50 1:500
41 / 1
1:50 1:1000
1:50 1:1500
1:100 1:200
1:100 1:500
1:100 1:1000
1:100 1:1500
1:200 1:500
1:400 1:500
1:50 1:200 41 / 2
1:50 1:500
1.2.4 Double Label Immunofluorescence
Double immunofluorescent labelling of the CB1 receptor with an antibody for NeuN, a
neuronal marker (monoclonal raised in mouse; Chemicon), was carried out for two reasons.
Firstly, the labelling pattern observed with immunohistochemical DAB staining needed to be
confirmed. Secondly, it was necessary to confirm that the CB1 receptor is expressed by
neurons. This protocol was the same as for the DAB immunohistochemistry, except for the
use of a fluorescent secondary antibody. The secondary antibodies utilised here were an Alexa
Fluor 488-conjugated anti-mouse IgG raised in goat (Invitrogen) for NeuN labelling and
Alexa Fluor 594-conjugated anti-goat IgG raised in donkey (Invitrogen) for the CB1 receptor.
The dilution of the CB1 receptor primary antibody was 1:100 and 1:1000 for the secondary
antibody. For NeuN, the primary antibody concentration was 1:200 and the secondary
antibody concentration was 1:500. Immunofluorescence reinforces the results obtained from
DAB immunohistochemistry as it completely eliminates the possibility of endogenous

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 130
peroxidase labelling within the tissue. Therefore, it reduces the possibility of non-specific
labelling to some extent, which in turn gives better end results. The CB1 receptor antibody
was incubated for two nights (41 hrs) whereas the NeuN antibody was incubated overnight
(both at 4o C). Because NeuN was a monoclonal antibody, blocking with serum was not
required. For both the CB1 receptor and NeuN primary antibodies, the secondary antibodies
were incubated for 1 hr at room temperature. As the secondary antibodies are light sensitive,
this procedure was carried out in the dark.
1.2.5 Data Acquisition
Unless stated otherwise, a Zeiss Axioplan MC80 BX microscope, Zeiss AxioCam HRc
digital camera and Zeiss Axiovision 3.1 software (Carl Zeiss Vision GmBH, Germany) were
employed for all morphological analyses and photography of immunohistochemical data.
Identical fields on each section stained with different antibody preparations were evaluated. In
this study, the dentate gyrus (DG) of the hippocampus was assessed. For visualising
fluorescent labelling, a Zeiss 510 LSM confocal microscope (Carl Zeiss GmbH, Germany)
and LSM 510 control software (version 3.2) were used.

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 131
1.3 Results
1.3.1 Optimisation of the CB1 Receptor Primary Antibody
In the first part of this study, CB1 receptor labelling with an antibody purchased from
Santa Cruz Biotechnology, Inc. was examined. Figure 1.1 shows the staining pattern obtained
with two different CB1 receptor primary antibody incubation times, to highlight the
differences in labelling observed. The incubation time window was selected on the basis of
previous immunohistochemical studies. From the literature, the standard CB1 receptor
primary antibody incubation time seems to be either overnight or 48 hrs (Tsou et al., 1998;
Ong and Mackie, 1999; Ashton et al., 2004; Fusco et al., 2004; Cristino et al., 2006; Grimsey
et al., 2008; Jelsing et al., 2008; Malone et al., 2008; Suàrez et al., 2008). In the present study,
18 hr (overnight) incubation appeared to be not sufficient to give CB1 receptor labelling,
therefore 41 hr incubation was chosen as an optimal primary antibody incubation duration
(Figure 1.1).
Figure 1.1
CB1 receptor labelling with different primary antibody incubation durations. The antibody
concentrations for both conditions were the same, i.e. 1:200 dilution for the primary antibody
and 1:500 dilution for the secondary antibody. Primary incubation durations were: (a) 18 and (b)
41 hrs. The secondary antibody was incubated for 1 hr for both primary incubation times. Scale
bars represent 100 m.
Figure 1.2 reveals how different antibody titres affect the CB1 receptor labelling. Similar
to antibody incubation time, there is a greater possibility of non-specific binding with higher
concentrations of the antibody. The staining with the 1:50 titre showed what may have been
some positively labelled cells for the CB1 receptor (Figure 1.2a). However, with a high
background this labelling cannot be guaranteed to be truly positive. On the other hand, the
1:400 titre showed too little CB1 receptor labelling (Figure 1.2d). Between 1:100 (Figure 1.2b)
a) 18 hrs b) 41 hrs

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 132
and 1:200 (Figure 1.2c) dilutions, the quality of the apparent CB1 receptor specific labelling
was equal, while the 1:100 titre had less background labelling. Therefore, a 1:100 dilution of
the CB1 antibody was selected as an optimal primary antibody concentration.
Figure 1.2
CB1 receptor labelling with different primary antibody concentrations. The secondary antibody
concentration (1:500 dilution) for all conditions remained constant. The dilutions for the
primary antibody were: (a) 1:50, (b) 1:100, (c) 1:200, (d) 1:400. The primary antibody was
incubated for 41 hrs and the secondary antibody for 1 hr. Scale bars represent 100 m.
a) 1:50 b) 1:100
c) 1:200 d) 1:400

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 133
1.3.2 Optimisation of the Secondary Antibody
The incubation time for the secondary antibody was much shorter than for the primary
antibody. In most studies, the secondary antibody is incubated for either 1 or 2 hrs at room
temperature (Tsou et al., 1998; Ong and Mackie, 1999; Fusco et al., 2004; Cristino et al.,
2006; De March et al., 2008; Grimsey et al., 2008; Jelsing et al., 2008; Suàrez et al., 2008).
Unlike the primary antibody, no particular change in labelling intensity or pattern was
observed when the two durations were tested (Figure 1.3). However, Figure 1.4 highlights the
fact that when compared to alteration of the primary antibody dilution, greater changes in the
intensity and the extent of labelling were observed as the secondary antibody dilution was
altered in various ways. Figure 1.4 shows that diluting the secondary antibody reduces the
level of background staining. When the 1:200 and 1:500 titres are compared to the 1:1000 and
1:1500 titres, it is apparent that there is less fine thread-like labelling in the background in the
1:1000 and 1:1500 titre group (Figure 1.4). However, when the antibody was diluted to
1:1500, it gave a negligible signal (Figure 1.4d). Therefore, a 1:1000 dilution was chosen as
an optimum secondary antibody concentration.
Figure 1.3
CB1 receptor labelling with different secondary antibody incubation durations. The primary and
the secondary antibody concentrations for both conditions were the same (1:50 and 1:500
dilution, respectively). Secondary antibody incubation durations were: (a) 1 and (b) 2 hrs. The
primary antibody was incubated for 41 hrs. Scale bars represent 100 m.
a) 1 hr b) 2 hrs

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 134
Figure 1.4
CB1 receptor labelling with different secondary antibody concentrations. The primary antibody
concentration remained constant for all conditions (1:100 dilution). The dilutions for the
secondary antibody were: (a) 1:200, (b) 1:500, (c) 1:1000, (d) 1:1500. The primary antibody was
incubated for 41 hrs and the secondary antibody for 1 hr. Scale bars represent 100 m.
a) 1:200 b) 1:500
c) 1:1000 d) 1:1500

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 135
1.3.3 Double Labelling Immunofluorescence
Double labelling of the CB1 receptor with a NeuN antibody suggested that the CB1
receptor is expressed by some of neurons in various regions of the hippocampus. The green
fluorescence represents neurons labelled with the NeuN antibody and red fluorescence
corresponds to cells apparently expressing the CB1 receptor (Figure 1.5). Apparent labelling
of the CB1 receptor was also observed in axons and dendrites (white arrows in Figure 1.5),
which was consistent in the DAB staining (Figure 1.6).
Figure 1.5
Double labelling immunofluorescence of the CB1 receptor antibody with an antibody for NeuN,
a neuronal marker, in the (a) CA1, (b) CA3, (c) hilus, (d) DG regions of the hippocampus. Green
fluorescence represents neurons and red fluorescence corresponds to cells expressing the
CB1 receptor. The white arrows indicate the axons and dendrites expressing the CB1 receptor.
The yellow arrows indicate neurons expressing the CB1 receptor. All scale bars represent 50
m.
a) CA1 b) CA3
c) Hilus d) DG

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 136
Figure 1.6
Immunohistochemical labelling of the CB1 receptor in axons and dendrites in the (a) stratum
oriens and (b) DG regions of the hippocampus. The primary antibody concentration was 1:100
and secondary antibody, 1:1000.
a) Stratum b) DG

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 137
1.4 Discussion
From the present study, an optimum protocol was obtained for using the Santa Cruz CB1
receptor antibody in the rat hippocampus. The optimum concentrations were 1:100 for the
CB1 receptor primary antibody and 1:1000 for the secondary antibody. Moreover, the
optimum incubation times for the primary and secondary antibodies were 41 hrs and 1 hr,
respectively. This study also proved that the antibody concentration and incubation time are
critical for the immunohistochemical experiments reported in this thesis (Appendix 2).
However, the western blot results (Chapter 4) suggested that the CB1 receptor antibody used
in the present study is not very specific. Therefore, although the present study showed
credible labelling of the CB1 receptors in the rat hippocampus, one cannot be confident that
this labelling is CB1 receptor-specific.
It was startling to see the difference in the apparent labelling of the CB1 receptors using
various conditions with the same batch of antibody from one commercial company. Should
the labelling be specific, the intensity of the staining must vary across the section for two
reasons: (1) the CB1 receptor antigen is not distributed evenly across the brain; and (2) the
brain tissue is sectioned at various levels of the cell. Under high magnification, the intensity
of false positive cells did not change throughout different regions of the brain tissue (data not
shown). With low CB1 primary antibody concentrations, the CB1 receptor antigen was not
recognised by the antibody, mainly because there were fewer available antibodies that could
bind to the antigen. The results from the present study suggest that while there would be
sufficient antibodies to recognise and bind to the antigen at the high concentration, it also
increases the level of the non-specific binding. Conversely, using a low concentration of the
primary antibody might decrease the non-specific binding, but the probability of obtaining
specific binding also decreases. Taken together, these results outline the importance of
determining an optimum primary antibody concentration for immunohistochemistry prior to
investigating changes in the CB1 receptor expression.
Optimising incubation duration is as important as finding the optimum antibody
concentration. Goldstein et al. (2007) demonstrated an altered immunohistochemical staining
percentage and intensity of estrogen receptor antibody labelling in invasive breast carcinomas,
by varying the antibody incubation time and the type of chromogen detection system.
Adequate time must be given for antibodies to bind to their specific antigen, yet if incubated
for too long, the chances of non-specific binding increase, which in turn will contribute to the

Appendix 1: Specificity of CB1 Receptor Labelling in the Hippocampus 138
elevation of staining intensity and the occurrence of false positive labelling. Typically, the
CB1 receptor antibody is incubated overnight or for 48 hrs. However, there is little published
in the literature regarding standardisation of the incubation time for the CB1 receptor antibody.
For this reason, the incubation time varies greatly between different immunohistochemical
studies. For example, one study stated that the CB1 receptor antibody was incubated for only
one hour (Pettit et al., 1998) and other studies did not even mention the antibody incubation
time (Egertova and Elphick, 2000; Moldrech and Wenger, 2000).
The present study clearly demonstrated that in immunohistochemistry, it is not only the
primary antibody which can cause non-specific binding, but also the secondary antibody to
some extent. Variations in the secondary antibody concentration resulted in variation in
immunohistochemical labelling. One possible explanation as to why the secondary antibody
contributes to greater non-specific binding than the primary antibody when the concentrations
of each were manipulated, is that when immunohistochemical staining is visualised, it is the
secondary antibody that converts or reacts with the chromogen to produce a visible coloured
product. Therefore, even if there is non-specific binding of the primary antibody, as long as it
is not bound by a secondary antibody, it cannot be observed. On the other hand, if the
secondary antibody is non-specifically bound to a protein it will give rise to coloured product
in the visualisation process. The current literature does not take into consideration how the
secondary antibody concentration can affect the immunohistochemical results. Because few
people are aware of how the secondary antibody concentration can affect the end result, many
of the immunohistochemical studies only control for the primary antibody. From the results of
this study, it is clear that the secondary antibody concentration must be taken into account as
an additional control when designing controls for antibody staining.
Although double labelling with a neuronal marker suggested that some of hippocampal
neurons express CB1 receptors, given the lack of specificity of the CB1 receptor primary
antibody, further studies are required to validate this result. Overall, the results from the
present study, together with the Grimsey et al (2008) study, cast considerable doubt upon the
binding specificity of the CB1 antibodies and indicate the need for standardising the
immunohistochemical procedure.

Appendix 2: Changes in CB1 Receptor Expression Following BVD 139
Appendix 2
Changes in CB1 Receptor Expression Following Bilateral
Vestibular Deafferentation in Rats
2.1 Introduction
A significant role of the endocannabinoid system in modulating hippocampal
neurotransmission was first suggested by the abundance of CB1 receptors observed in this
brain region using radioligand binding assays and autoradiography (Devane et al., 1988;
Herkenham et al., 1990, 1991) and in situ hybridisation of receptor mRNA (Mailleux and
Vanderhaeghen, 1992; Matsuda et al., 1993). To date, there have been several
immunohistochemical studies evaluating the distribution and localisation of the CB1 receptors
in the brain (Pettit et al., 1998; Tsou et al., 1998; Ong and Mackie, 1999; Moldrich and
Wenger, 2000; Egertová and Elphick, 2000; Suárez et al., 2008). Although current findings
demonstrate both the presence and the level of CB1 receptor expression in the hippocampus,
there is a lack of information regarding the spatial distribution of the CB1 receptors across the
hippocampus.
The most accurate method for measuring the spatial distribution of biological objects
within an anatomical structure is unbiased stereology (see Glaser and Glaser, 2000;

Appendix 2: Changes in CB1 Receptor Expression Following BVD 140
Mamdarim de Lacerda et al., 2010 for review). This method is currently the most advanced
and the most modern for quantifying immunohistochemical labeling, as it generates accurate
cell numbers within anatomically defined structures (West and Gundersen, 1990; West et al.,
1991; Abusaad et al., 1999; Keuker et al., 2001; Hosseini-Sharifabad and Nyengaard, 2007).
However, this approach involves the counting of immuno-positive cells from sampled
sections, which is both labour-intensive and time-consuming. For this reason, the number of
studies using stereological approaches to determine the spatial distribution of a particular
receptor type (Miettinen et al., 2002; Zhang et al., 2005a, b; Price et al., 2009), is relatively
small compared with studies reporting the presence of that receptor by using other
biochemical assays such as western blotting.
There are several methods other than stereology by which immunohistochemical
staining may be meaningfully analysed and quantified. A scoring system has been used by
some researchers (Detre et al., 1995; Koethe et al., 2007; Suàrez et al., 2008; Sutherland et al.,
2009), while others simply report the extent and intensity of the immunohistochemical
labeling by visually identifying qualitative changes in receptor expression (Mitrirattanakul et
al., 2007; Ludanyi et al., 2008; Falenski et al., 2007, 2009; Araujo et al., 2010). However,
these arbitrary interpretations of immunohistochemical staining by classifying labelling as
„weak, moderate or strong‟ are observer-based, and therefore, subjective. No matter how
clearly the criteria are set forth, the application of such criteria inevitably results in variation
in outcomes between observers. In order to overcome this variability and to ensure more
objective analysis, computer-assisted image analyses have been utilised (Kim et al., 1997;
Simantov et al., 1999; Williams et al., 2005; Malone et al., 2008; Dowie et al., 2009; Suàrez
et al., 2009; Walsh et al., 2010).
To date, there has been no study investigating changes in CB1 receptor expression in the
hippocampus or in other brain regions following BVD. While Ashton and others have
examined CB1 receptor expression in the hippocampus, this investigation was following UVD
and there were no statistically significant differences in UVD animals compared to the control
groups (Ashton et al., 2004b). Furthermore, the majority of the studies which have
investigated the effects of peripheral vestibular damage on the expression of various receptor
types in the brain have used western blotting to quantify the level of expression (Li et al.,
1997; King et al, 2002; Horii et al., 2003; Lindsay et al., 2005). Although western blotting
analysis provides some measure of receptor density, the spatial distribution of the expression
cannot be obtained. Therefore, the aim of the present study was to utilise

Appendix 2: Changes in CB1 Receptor Expression Following BVD 141
immunohistochemistry and computer-assisted image analysis to determine whether BVD
surgery alters the spatial distribution of the CB1 receptors in different sub-regions of the rat
hippocampus. However, due to concerns about the specificity of the CB1 receptor antibody
(Grimsey et al., 2008; also see Chapter 4), the results obtained from the present study are
uncertain.

Appendix 2: Changes in CB1 Receptor Expression Following BVD 142
2.2 Materials and Methods
2.2.1 Animals
Thirty-six naïve male Wistar rats were used, each weighing 250-300 g at the time of the
surgery. There were 3 post-operative time points; 1, 3, and 7 days. For each time point, 6
sham and 6 BVD animals were used. All animals were decapitated without anaesthesia. The
brains were rapidly removed, fixed and sectioned as described in Sections 1.2.1 and 1.2.2 in
Appendix 1, respectively.
2.2.2 Immunohistochemistry
The sagittal hippocampal sections were labelled for the CB1 receptors as described in
Section 1.2.3 of Appendix 1. Briefly, the concentrations of the CB1 receptor primary antibody
and donkey anti-goat secondary antibody used were 1:100 and 1:1000, respectively.
2.2.3 Data Acquisition
A Zeiss Axioplan MC80 BX microscope, Zeiss AxioCam HRc digital camera and Zeiss
Axiovision 3.1 software (Carl Zeiss Vision GmBH, Germany) were employed to obtain
immunohistochemical images. The CB1 receptor labelling quantification was carried out by
measuring the grey level density from digital images using the ImageJ 1.38x analysis
programme (Wayne Rasband, National Institutes of Health, USA, http://rsb.info.nih.gov/ij/).
2.2.4 Statistical Analysis
Statistical analyses were performed in SPSS 17. Two-way ANOVAs with repeated
measures were carried out to determine changes in the spatial distribution of the CB1
receptors across the hippocampus in a sagittal direction. The area under the curve (AUC) was
calculated from the spatial distribution graphs (Prism 5) to determine the effect of post-
operative time (1, 3, or 7 days) on CB1 receptor expression. In order to determine whether
CB1 receptor expression was different between the dorsal and ventral hippocampus, the AUC
was calculated in a dorsal-ventral direction. Then two-way ANOVAs were performed with
surgery and anatomical position (that is, dorsal and ventral) as factors.

Appendix 2: Changes in CB1 Receptor Expression Following BVD 143
2.3 Results
2.3.1 Spatial Distribution of the CB1 Receptors in the Hippocampus
Representative immunohistochemical sections from the sham and BVD animals in
Figure 2.1 exemplify changes observed in CB1 receptor expression in the hippocampus. For
the analysis in which sagittal sections were compared in a medio-lateral direction, there was a
significant surgery effect at 1 day post-operation in the CA1 and CA3 areas of the
hippocampus, where BVD animals‟ CB1 receptor expression was higher in the CA1 area and
lower in the CA3 area compared to the sham animals (F(1,123) = 7.54; P = 0.007 for CA1;
F(1,116) = 7.18; P = 0.008 for CA3; Figure 2.2a, b). Furthermore, the CB1 receptor expression
decreased across the DG region of the hippocampus at 7 days post-operation in the medio-
lateral plane (F(8,90) = 2.86; P = 0.007; Figure 2.2i). There were no significant interactions
between the surgery and anatomical positions in any regions or at any time points (Figure 2.2).
Figure 2.1
Representative immunohistochemical sections of the CB1 receptor labelling in the
hippocampus of (a) a sham and (b) a BVD animal. The scale bars represent 100 m.
a) SHAM b) BVD

Appendix 2: Changes in CB1 Receptor Expression Following BVD 144
Figure 2.2
The spatial distribution of the CB1 receptors across the CA1, CA3, and DG of the hippocampus
at (a – c) 1, (d – f) 3, and (g – i) 7 days following BVD compared to the sham controls. Sections
were analysed in a medio-lateral direction. Data are represented as mean + SEM. P values are
shown where there was a significant effect.
1 day post-operation 3 days post-operation 7 days post-operation
CA
1
CA
3
DG
a) b) c)
d) e) f)
g) h) i)

Appendix 2: Changes in CB1 Receptor Expression Following BVD 145
The ventral hippocampus of both the sham and BVD animals showed significantly
lower CB1 receptor expression compared to the dorsal region at 1 and 7 days post-operative
time points (1 day, F(1,68) = 11.57, P = 0.001, Figure 2.3a; 7 days, F(1,68) = 4.26, P = 0.043,
Figure 2.3c), but not at 3 days (F(1,68) = 0.78, P = 0.381, Figure 2.3b). There was no significant
surgery effect or interaction between surgery and anatomical position at any post-operative
time points (Figure 2.3).
Figure 2.3
The AUC of the spatial distribution of CB1 receptors in the dorsal and ventral hippocampus at
(a) 1, (b) 3, and (c) 7 days following BVD compared to the sham controls. Data are represented
as mean + SEM.
a) b)
c)

Appendix 2: Changes in CB1 Receptor Expression Following BVD 146
2.4 Discussion
The results from the present study suggest apparent changes in the expression of the
CB1 receptors following BVD in specific sub-regions of the hippocampus. These changes
include a significant increase in CB1 receptor expression in the CA1 region in BVD rats
compared to sham controls at 1 day post-operation, concurrent with a significant reduction in
the CA3 region of BVD compared to sham animals, with no change in the DG. Although a
reduction in the expression of the CB1 receptors in the CA3 region observed in the present
study is superficially consistent with the western blot data (Chapter 4), due to the lack of CB1
receptor antibody specificity shown by the western blotting (Chapter 4; Grimsey et al., 2008),
no definite conclusion cannot be drawn other than that the CB1 receptor antibody is in doubt.

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 147
Appendix 3
The Specificity of Cannabinoid CB2 Receptor Labelling in
the Rat Hippocampus
3.1 Introduction
In 1993, Munro et al. discovered a second cannabinoid receptor subtype, the so-called
CB2 receptor, that does not mediate the psychoactive effects of cannabinoids. The initial
studies that explored the distribution of this „peripheral cannabinoid receptor‟ indicated that
CB2 receptors are present exclusively in the periphery, particularly in tissues and cells of the
immune system, such as spleen macrophages, tonsils, B cells and natural killer cells,
monocytes, neutrophils and T cells (see Fernández-Ruiz et al., 2007 for review). Being absent
from the CNS (Lynn and Herkenham, 1994), the distribution of this second cannabinoid
receptor was in contrast with the well-known distribution of the CB1 receptor. However,
further studies investigating the expression of CB2 receptors have raised significant
controversy regarding their presence in the mammalian brain. While CB2 receptors have not
been found in the intact CNS by some researchers (Derocq et al., 1995; Galiègue et al., 1995;
Schatz et al., 1997; Griffin et al., 1999; Sugiura et al., 2000), others have shown that CB2
receptor expression might be induced in glial cells, in particular reactive microglia, in
response to diseases, such as Alzheimer‟s disease (Benito et al., 2003; Ramirez et al., 2005),

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 148
brain tumours (Ellert-Miklaszewska et al., 2007), multiple sclerosis (Benito et al., 2007), and
Parkinson‟s disease (Price et al., 2009).
Despite the fact that numerous studies have failed to detect CB2 receptors in healthy
brains, recent studies have reported the expression of CB2 receptors in both cultured neural
cells and in the nervous system of several mammals under normal conditions. For example,
CB2 receptors have been reported to be expressed in glial cells, including microglia and
astrocytes (Núñez et al., 2004; Arévalo-Martín et al., 2007), endothelial cells (Ashton et al.,
2006), neural progenitors (Palazuelos et al., 2006), neural stem/precursor cells (Molina-
Holgado et al., 2007), and most recently in the sub-regions of the hippocampus (Suàrez et al.,
2009). Only a few years ago, the answer to the question of whether CB2 receptors are
expressed in neurons was clearly „no‟. However, recent evidence has opened this field to
further discussion.
CB2 receptor mRNA expression has been reported in cerebellar neurons (Skaper et al.,
1996) and more recently, Van Sickle et al. (2005) reported CB2 receptor mRNA and protein in
the cerebellum, cortex and brainstem neurons of the rat, the mouse, and the ferret. In addition,
Gong and others have demonstrated abundant expression of CB2 receptor mRNA and protein
in numerous regions of the intact brain (Gong et al., 2006), and Suàrez et al. (2008) identified
CB2 receptor expression in neurons of the cerebellum and the brainstem vestibular nucleus,
which was replicated by Baek et al. (2008). Furthermore, evidence for a functional role of
CB2 receptors was reported by Jhaveri et al. (2008) in thalamic neurons and by Morgan et al.
(2009) in hippocampal neurons.
Nevertheless, the expression of CB2 receptors in the brain remains controversial due to
uncertainty about the experimental approaches used, and the specificity of some
methodological tools such as CB2 receptor antibodies. At present, no previous study has
compared the specificity of CB2 receptor antibodies from different commercial companies. As
has been shown by Grimsey et al. (2008) and Jelsing et al. (2008), the specificity of
antibodies can vary immensely, and these authors emphasise the importance of thorough
validation of the specificity of antibodies for each particular application before use. There are
many possible factors which could affect the labelling pattern of the same target antigen by
different „specific‟ antibodies. Some of these include the method of tissue fixation, tissue
thickness, antigen retrieval, antibody concentration, incubation duration and temperature.

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 149
Although Gong et al. (2006) compared different CB2 receptor primary antibodies from
three commercial companies, Cayman, Sigma, and Santa Cruz Biotechnology (Santa Cruz),
they did not attempt to alter antibody dilution or the incubation time for any of the primary or
secondary antibodies. Even though they used controls such as a blocking peptide, a brain
section from CB2 receptor knockout mice, and a control section incubated without the primary
antibody, the use of antibodies from different companies was not consistent throughout the
experimental design. For example, only the antibody from Santa Cruz, but from no other
manufacturer, was used to analyse the specificity of the antibody in knockout mice brain
tissue sections. Also, a blocking peptide was used only with the antibody from Cayman.
Further controversy regarding CB2 receptor labelling was revealed when Suàrez et al. (2008)
reported that cerebellar Purkinje cell bodies were not CB2 receptor immunopositive, when the
same type of cells had been shown to be intensely stained for CB2 receptors in the Gong et al.
(2006) study. The astounding differences in the labelling pattern of CB2 receptors observed in
the above studies raise serious concerns about the specificity of the CB2 receptor antibodies,
not only for the above studies, but for any study which has used CB2 receptor antibodies to
localise CB2 receptor expression in the brain using immunohistochemistry.
Numerous studies using immunohistochemistry may have reached invalid conclusions
due to misinterpretation of non-specific staining patterns. Moreover, the limitations of using
antibodies to perform immunohistochemistry, especially on brain tissue sections, are said to
be greater than for other biochemical methods (Rhodes and Trimmer, 2006). The main reason
for this is that the brain has a relatively complex and heterogeneous cellular and molecular
composition compared to other tissues. Because immunoreactivity in immunohistochemistry
reveals all sites to which the antibodies bind, it leaves considerable room for misinterpretation
and confusion as to which labelling is specific and which is non-specific. One approach to
obtain concordant data, suggested by Rhodes and Trimmer (2006), is to compare the labelling
patterns revealed by two different antibody preparations raised against distinct, non-
overlapping antigenic sequences on the same target protein. This is important because
different antibodies binding to different epitopes of the target protein may result in different
labelling patterns. At present, many commercial companies are manufacturing CB2 receptor
primary antibodies. All of the CB2 receptor primary antibodies that are currently available
bind to either the N- or C-terminus of the CB2 receptor protein (Figure 3.1). Furthermore,
since there are no monoclonal CB2 receptor antibodies that are commercially available at
present, one should be cautious obtaining data using currently available polyclonal CB2
receptor antibodies, because the specificity of such polyclonal antibodies may vary over time

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 150
depending on the breed and on the individual animals being immunised during antibody
production (Leenaars & Hendriksen, 2005).
Figure 3.1
A CB2 receptor diagram showing the locations of different commercial and privately made CB2
receptor primary antibodies targeting different epitopes of the CB2 receptor protein. All except
the Ken Mackie antibody are manufactured by commercial companies.
Overall, it has been increasingly recognised by many researchers how difficult it is to
obtain a true representation of an antigen of interest using a specific antibody in
immunohistochemistry. Furthermore, the precise conditions for the immunohistochemical
assay can make an enormous difference to the results (Goldstein et al., 2007; Lorincz and
Nusser, 2008). Therefore, it is very important to have sufficient controls in order to convince a
sceptical observer. The present study aimed to investigate the specificity of two commercially
available polyclonal CB2 receptor antibodies by varying the concentrations and durations of
exposure used for the primary and secondary antibodies. Although it was initially intended to
analyse the expression of CB2 receptors in the hippocampus following BVD, concerns about
antibody specificity resulted in these experiments being discontinued.
Abcam (aa 1-32)
Sigma (aa 1-33)
Affinity BioReagents (aa 1-33) Cayman
(aa 20-33)
Alpha Diagnostics
& Santa Cruz
(C-terminus)
Ken Mackie
(aa 326-342)
Santa Cruz
(N-terminus)

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 151
3.2 Materials and Methods
3.2.1 Animals
Three male Wistar rats were used for immunohistochemistry and 2 for the western
blotting. Each animal weighed 250-300 g. The procedure of harvesting and sectioning the
brain tissue was the same as described in Sections 1.2.1 and 1.2.2 of Appendix 1. The tissue
dissection and homogenisation procedures for western blotting are described in Sections
4.2.1.2 and 4.2.1.3 of Chapter 4.
3.2.2 Western blotting
Western blotting procedures for the CB2 receptor were the same as described in Section
4.1.2.3 of Chapter 4. The CB2 receptor primary antibody concentration for both the Santa
Cruz and the Abcam antibodies was 1:1000 and their corresponding secondary antibody
concentration was 1:5000. The HRP-conjugated donkey anti-goat secondary antibody was
used for the Santa Cruz CB2 receptor primary antibody and the HRP-conjugated goat anti-
rabbit secondary antibody was used for the Abcam CB2 receptor primary antibody.
3.2.3 Immunohistochemistry
Immunohistochemical procedures for the labelling of the CB2 receptor were the same as
for labelling the CB1 receptors, as described in Section 1.2.3 of Appendix 1. Both the primary
(goat polyclonal CB2 receptor) and the secondary (HRP-conjugated donkey anti-goat)
antibodies used in this experiment were from Santa Cruz, Inc.
In order to obtain the best possible immunohistochemical labelling of the CB2 receptors,
various combinations of both primary and secondary antibody concentrations and incubation
durations were tested (Table 2.1). Briefly, the primary antibody dilutions ranged from 1:50 to
1:400 and the incubation was either overnight (18 hrs) or for 2 nights (41 hrs). The secondary
antibody concentrations varied from 1:500 to 1:1000 dilutions, and the incubation durations
were either 1 or 2 hrs.

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 152
Table 3.1
Various combinations of CB2 receptor primary (1o) and subsequent secondary (2
o) antibody
titres trialled and their incubation durations.
1o antibody
concentration
2o antibody
concentration
1o / 2
o antibody incubation
time (hrs)
1:200 1:500 41 / 1
1:400
1:50
1:1000 41 / 1 1:100
1:200
1:200 1:500
41 / 1 1:1000
1:200 1:500
18 / 1
41 / 1
41 / 2
3.2.4 Double Label Immunofluorescence
Double immunofluorescent labelling of the CB2 receptor (Santa Cruz) with a neuronal
marker, NeuN (mouse monoclonal, Chemicon) was carried out in order to further characterise
the types of cell(s) in which CB2 receptors are expressed in the brain. The primary antibody
concentration for both the CB2 receptor and NeuN was 1:200, and for subsequent secondary
antibodies, 1:500. The Alexa Fluor 594-conjugated donkey anti-goat IgG (Invitrogen) resulted
in red fluorescence for CB2 receptor positive cells and the Alexa Fluor 488-conjugated goat
anti-mouse IgG (Invitrogen) resulted in green fluorescence for NeuN labelling. The CB2
receptor antibody was incubated for 41 hrs and NeuN was incubated overnight, both at 4o C.
Because NeuN is a monoclonal antibody, blocking with serum was not required. For both the
CB2 receptor and NeuN antibodies, the secondary antibodies were incubated for 1 hr at room
temperature. As the secondary antibodies are light sensitive, this procedure was carried out in
the dark.
Due to the use of a different CB2 receptor primary antibody in double labelling (Abcam)
compared with the single labelling (Santa Cruz), double labelling using both of these CB2
receptor primary antibodies was also performed. An Alexa Fluor 488-conjugated donkey anti-
goat IgG antibody (Invitrogen) was used as a secondary antibody for the CB2 goat polyclonal
antibody from Santa Cruz. Both primary and secondary antibody concentrations and

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 153
incubation durations were kept the same as for the single labelling protocol. The purpose of
this experiment was to determine whether there were any differences in the labelling pattern
with antibodies from two different commercial companies, which nominally target the same
antigen (both N-terminus).
3.2.5 Data Acquisition
Unless stated otherwise, a Zeiss Axioplan MC80 BX microscope, Zeiss AxioCam HRc
digital camera and Zeiss Axiovision 3.1 software (Carl Zeiss Vision GmBH, Germany) were
employed for all morphological analyses and photography of immunohistochemical data.
Identical fields on each section stained with different antibody preparations were evaluated. In
this study, various regions of the hippocampus were assessed. For visualising fluorescent
labelling, a Zeiss 510 LSM confocal microscope (Carl Zeiss GmbH, Germany) and LSM 510
control software (version 3.2) running on a Windows PC was used.

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 154
3.3 Results
3.3.1 Western Blotting
In spleen tissue, the CB2 antibody from Santa Cruz produced two very weak bands
around 30 kDa and the CB2 antibody from Abcam produced multiple bands within the 30 – 45
kDa range (Figure 3.2, rectangle box). In the hippocampal tissues, both the Santa Cruz and
Abcam CB2 receptor antibodies labelled multiple protein bands in the molecular weight range
of 32 – 45 kDa (Figure 3.2, rectangle box). However, the molecular weight of the bands in the
hippocampal tissue did not match that in the spleen tissue. Furthermore, labelling of many
other protein bands could be seen with both antibodies in both the spleen and the hippoampal
tissues (Figure 3.2).
Figure 3.2
Western blots of hippocampal tissue (Hp1 and Hp2) and spleen (sp, as a positive control),
using the Santa Cruz and Abcam CB2 receptor antibodies. The rectangle indicates the
molecular weight range for the CB2 receptor protein.
3.3.2 Immunohistochemical Analysis of the CB2-Specific Antibody
Figure 3.3 shows the staining pattern obtained with two different CB2 receptor primary
antibody concentrations to highlight the differences in the specificity. In order to examine the
effect of altering the primary antibody concentrations, the secondary antibody concentration
was maintained at the dilution of 1:500 for both sections. It was clear that compared to a
1:200 concentration (Figure 3.3a), a 1:400 dilution of the CB2 receptor primary antibody was

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 155
too weak to obtain any specific labelling in the hippocampus (Figure 3.3b). Although when
compared to a 1:200 concentration, staining with a 1:400 concentration could be mistaken for
a negative control, when compared to a true negative control section (Figure 3.3c), there was
clearly less staining in the negative control than with a 1:400 antibody concentration.
Figure 3.3
Immunohistochemical comparison of CB2 receptor labelling with different primary antibody
concentrations in the dentate gyrus (DG) of the rat hippocampus. The secondary antibody
concentration for all conditions was the same, i.e. 1:500. The dilutions for the primary antibody
were: (a) 1:200, (b) 1:400, (c) negative control. The primary antibody was incubated for 41 hrs,
the secondary antibody for 1 hr. Scale bars indicate 100 m.
A lower secondary antibody concentration (1:1000) was tested with various
concentrations of the primary antibody (1:50, 1:100, or 1:200; Figure 3.4). Similar results to
those shown in Figure 3.3 were obtained: as the concentration of the primary antibody
decreased, the antibody specificity decreased also. Although 1:50 primary and 1:1000
secondary antibody dilution combinations gave similar results to a 1:200 primary and 1:500
secondary combination, overall, less antibody was required for the latter combination. These
results indicate that it is very important to determine the optimum primary antibody
concentration.
a) 1:200
b) 1:400 c) Negative control

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 156
Figure 3.4
Immunohistochemical comparison of CB2 receptor labelling with different primary antibody
concentrations in the DG of the rat hippocampus. The secondary antibody concentration was a
1:1000 dilution. The dilutions for the primary antibody were: (a) 1:50, (b) 1:100, (c) 1:200. The
primary antibody was incubated for 41 hrs, the secondary antibody for 1 hr. Scale bars indicate
100 m.
Having investigated the optimal primary antibody concentration, the secondary antibody
concentrations were then investigated. Figure 3.5 shows that varying the concentration of the
secondary antibody alters the final outcome. The primary antibody concentration was kept
constant at a 1:200 titre, whereas two different concentrations of the secondary antibody were
tested: 1:500 and 1:1000 dilutions. A 1:500 dilution gave apparent specific labelling of the
CB2 receptor (Figure 3.5a), whereas a 1:1000 dilution was inadequate (Figure 3.5b).
Figure 3.5
Immunohistochemical comparison of CB2 receptor labelling with different secondary antibody
concentrations in the DG of the rat hippocampus. The primary antibody concentration for all
conditions was constant at a 1:200 dilution. The dilutions for the secondary antibody were: (a)
1:500, (b) 1:1000. The primary antibody was incubated for 41 hrs and the secondary antibody
for 1 hr. Scale bars indicate 100 m.
a) 1:50
b) 1:100 c) 1:200
a) 1:500 b) 1:1000

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 157
Not only the antibody concentration, but also the incubation duration, is important for
specific labelling in immunohistochemistry (Goldstein et al., 2007). Figure 3.6a and b show
the effect of different primary antibody incubation durations, while the primary and secondary
antibody concentrations were kept the same. Surprisingly, an overnight (18 hr) incubation
duration for the CB2 receptor primary antibody (Figure 3.6a) was not sufficient to allow what
appears to be specific binding to occur. However, when incubated for 2 nights (41 hrs),
apparent specific labelling was achieved (Figure 3.6b). Unlike primary antibodies, alteration
of the secondary antibody incubation duration did not alter the apparent specific binding
(Figure 3.6b, c). Figure 3.7 shows apparent specific labelling of the CB2 receptors in different
areas of the hippocampus. CB2 receptor-positive labelling is clearly visible in the cytoplasm,
nucleus, nuclear membrane, and cell membrane. Dendrites were also labelled for CB2
receptors, particularly in the CA3 area of the hippocampus (Figure 3.7c).
Figure 3.6
Immunohistochemical comparison of CB2 receptor labelling with different antibody incubation
durations in the DG of the rat hippocampus. The primary and secondary antibody
concentrations in all conditions were the same at 1:200 for the primary antibody and 1:500 for
the secondary antibody. Antibody incubation durations were (primary/secondary): (a) 18/1 hrs,
(b) 41/1 hrs, (c) 41/2 hrs. Scale bars indicate 100 m.
a) 18/1 hrs
c) 41/2 hrs
b) 41/1 hrs

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 158
Figure 3.7
Immunohistochemical labelling of CB2 receptors in the hippocampus. Positive
immunoreactivity to the CB2 receptor was expressed as brown staining in the cytoplasm
(purple arrow), nucleus (blue arrow), nuclear membrane (red arrow), and cell membrane (green
arrow). Low magnification image of the entire hippocampus is shown in (a). Higher
magnification images of various regions of the hippocampus: (b) CA1, (c) CA3, (d) DG. The
dilution for the CB2 receptor primary antibody was 1:200 and for the secondary antibody 1:500.
The primary antibody was incubated for 41 hrs at 4 o
C and the secondary for 1 hr at room
temperature. Scale bars for (a) and (b-d) indicate 200 and 20 m, respectively.
3.3.3 Double Labelling Immunofluorescence
Double immunofluorescence labelling showed the apparent co-localisation of the CB2
receptor and the NeuN antibodies in the CA3 region of the hippocampus (Figure 3.8a, b).
However, there was no apparent co-localisation of the CB2 receptor and neurons in the dentate
gyrus (DG) area of the hippocampus (Figure 3.8c).
Double labelling of two CB2 receptor antibodies from different commercial companies
(Abcam and Santa Cruz) showed surprising results. The overall labelling pattern of these two
antibodies appeared to be surprisingly similar with abundant immunofluorescence observed in
a) Hippocampus b) CA1
c) CA3 d) DG

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 159
CA3 (Figure 3.9a, b). In the same section, the Santa Cruz antibody labelled the cytoplasm,
nucleus, nuclear membrane, and cell membrane (Figure 3.9) which matched the result
obtained from DAB single labelling of the CB2 receptors (Figure 3.7), while the Abcam
antibody labelled only the cytoplasm of the same cell (Figure 3.9c).
Figure 3.8
Double labelling immunofluorescence of the CB2 receptor antibody with a NeuN antibody, a
neuronal marker in various regions of the hippocampus: (a) CA3, (b) CA3 at higher
magnification, (c) DG. Green fluorescence represents neurons and red fluorescence
corresponds to cells expressing the CB2 receptor. The white arrows indicate NeuN-positive
neurons, yellow arrows indicate neurons expressing the CB2 receptor, and blue arrows indicate
cells expressing only the CB2 receptor. Scale bars for (a, c) and (b) indicate 50 and 20 m,
respectively.
a) CA3 b) CA3
c) DG

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 160
Figure 3.9
Immunofluorescent labelling showing the distribution of the CB2 receptors in the CA3 region of
the hippocampus using antibodies from the (a) Abcam (rabbit polyclonal) and (b) Santa Cruz
(goat polyclonal). (c) Double labelling of CB2 receptors using antibodies from the Santa Cruz
(Green) and Abcam (Red). Scale bars for (a, b) and (c) indicate 50 and 20 m, respectively.
a) Abcam b) Santa Cruz
c) Abcam/Santa Cruz

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 161
3.4 Discussion
From the present study, the optimum procedures for the use of the Santa Cruz CB2
receptor antibody in immunohistochemistry were determined, which were a 1:200 dilution for
the CB2 receptor primary antibody, and a 1:500 dilution for the secondary antibody
concentrations, with 41 hrs and 1 hr as the optimum incubation times for the primary and
secondary antibodies, respectively. However, consistent with the CB1 receptor antibodies
(Appendix 1), variation in the CB2 receptor primary antibody and secondary antibody
concentrations and incubation durations did affect the CB2 receptor antibody labelling in the
brain. Fortunately, the same Santa Cruz antibody used in this study was previously used by
Ashton and others (Ashton et al., 2006, 2007). In Ashton et al. (2006), the primary antibody
concentration was 1:50 and the secondary antibody concentration was 1:1000. This
combination was tested in the current study and it was found to give equivalent labelling to
that reported by Ashton et al. (2006).
Unlike the CB1 receptor (Grimsey et al., 2008; Jelsing et al., 2008), the specificity of
the CB2 receptor antibodies used in immunohistochemical studies has not been investigated
thoroughly. In addition, because most of the antibodies are capable of producing non-specific
binding, there is still controversy surrounding the specificity of the CB2 receptor antibodies.
Therefore, it is crucial to systematically evaluate the specificity of an antibody in order to
obtain reliable data. The western blotting showed that both the Santa Cruz and Abcam CB2
receptor antibodies labelled multiple protein bands in addition to those in the molecular
weight range for the CB2 receptor protein. This was similar to the results obtained for
commercial CB1 receptor antibodies by Grimsey et al. (2008). Most importantly, the spleen
tissue which is known to have high levels of CB2 receptors failed to show consistent band(s)
when probed with these two antibodies. Based on these results, non-specific labelling using
immunochemistry with these antibodies would be expected.
In order to address this problem, the current study used not only two different CB2
receptor antibodies, but also carried out double labelling of the CB2 receptor antibody with a
neuronal marker, NeuN. It was shown that the CB2 receptor antibody from Abcam appears to
label neurons in the hippocampus. The specificity was further investigated by the double
labelling of two CB2 receptor antibodies which were from Abcam and Santa Cruz. It was
interesting to see the difference in the labelling pattern. The Abcam antibody labelled the
cytoplasm and cell membrane, whereas the Santa Cruz antibody stained the entire cell. The

Appendix 3: Specificity of CB2 Receptor Labelling in the Hippocampus 162
Santa Cruz antibody labelling was consistent with Ashton et al. (2006, 2007). On the other
hand, the cytoplasmic labelling pattern of the Abcam antibody was observed in the Van Sickle
et al. (2005) study, in which the CB2 receptor antibody used was from Alpha Diagnostics.
Taken together, the results from the present study suggest that CB2 receptor “specific”
antibodies are likely to produce non-specific labelling in immunohistochemistry. This
undermines the idea of CB2 receptor expression in the hippocampus. Therefore, great caution
needs to be taken when interpreting the results of brain immunohistochemistry using some
commercial CB2 receptor antibodies.

163
References

References 164
Abel, E.L. (1971) Retrieval of information after use of marihuana. Nature, 231, 58.
Abusaad, I., MacKay, D., Zhao, J., Stanford, P., Collier, D.A. & Everall, I.P. (1999) Stereological estimation of
the total number of neurons in the murine hippocampus using the optical disector. J Comp Neurol, 408,
560-566.
Abush, H. & Akirav, I. (2009) Cannabinoids modulate hippocampal memory and plasticity. Hippocampus, 20,
1126-1138.
Adams, I.B. & Martin, B.R. (1996) Cannabis: pharmacology and toxicology in animals and humans. Addiction,
91, 1585-1614.
Adermark, L. & Lovinger, D.M. (2007) Combined activation of L-type Ca2+ channels and synaptic transmission
is sufficient to induce striatal long-term depression. J Neurosci, 27, 6781-6787.
Adermark, L., Talani, G. & Lovinger, D.M. (2009) Endocannabinoid-dependent plasticity at GABAergic and
glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci, 29, 32-41.
Aggleton, J.P. (1985) One-trial object recognition by rats. Q J Exp Psychol B, 37, 279-294.
Agu, R.U., Valiveti, S., Paudel, K.S., Klausner, M., Hayden, P.J. & Stinchcomb, A.L. (2006) Permeation of WIN
55,212-2, a potent cannabinoid receptor agonist, across human tracheo-bronchial tissue in vitro and rat
nasal epithelium in vivo. J Pharm Pharmacol, 58, 1459-1465.
Aigner, T.G. (1988) Delta-9-tetrahydrocannabinol impairs visual recognition memory but not discrimination
learning in rhesus monkeys. Psychopharmacology (Berl), 95, 507-511.
Allen, K., Potvin, O., Thibaudeau, G., Dore, F.Y. & Goulet, S. (2007) Processing idiothetic cues to remember
visited locations: hippocampal and vestibular contributions to radial-arm maze performance. Hippocampus,
17, 642-653.
Allen, K.L., Waldvogel, H.J., Glass, M. & Faull, R.L. (2009) Cannabinoid (CB(1)), GABA(A) and GABA(B)
receptor subunit changes in the globus pallidus in Huntington's disease. J Chem Neuroanat, 37, 266-281.
Allum, J.H. & Pfaltz, C.R. (1985) Visual and vestibular contributions to pitch sway stabilization in the ankle
muscles of normals and patients with bilateral peripheral vestibular deficits. Exp Brain Res, 58, 82-94.
Altemus, K.L. & Almli, C.R. (1997) Neonatal hippocampal damage in rats: long-term spatial memory deficits
and associations with magnitude of hippocampal damage. Hippocampus, 7, 403-415.
Amaral, D. & Lavenex, P. (2007) Hippocampal neuroanatomy. In Anderson, P., Morris, R., Amaral, D., Bliss, T.
& O'Keefe, J. (ed) The Hippocampus Book. Oxford University Press, New York, pp. 37-114.
Ameri, A. (1999) The effects of cannabinoids on the brain. Prog Neurobiol, 58, 315-348.
Ames, F. (1958) A clinical and metabolic study of acute intoxication with Cannabis sativa and its role in the
model psychoses. J Ment Sci, 104, 972-999.
Angelaki, D.E. & Cullen, K.E. (2008) Vestibular system: the many facets of a multimodal sense. Annu Rev
Neurosci, 31, 125-150.
Angelaki, D.E., Klier, E.M. & Snyder, L.H. (2009) A vestibular sensation: probabilistic approaches to spatial
perception. Neuron, 64, 448-461.
Araujo, B.H., Torres, L.B., Cossa, A.C., Naffah-Mazzacoratti Mda, G. & Cavalheiro, E.A. (2010) Hippocampal
expression and distribution of CB1 receptors in the Amazonian rodent Proechimys: an animal model of
resistance to epilepsy. Brain Res, 1335, 35-40.
Arenos, J.D., Musty, R.E. & Bucci, D.J. (2006) Blockade of cannabinoid CB1 receptors alters contextual

References 165
learning and memory. Eur J Pharmacol, 539, 177-183.
Arévalo-Martín, A., Garcia-Ovejero, D., Rubio-Araiz, A., Gomez, O., Molina-Holgado, F. & Molina-Holgado, E.
(2007) Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the
subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur J
Neurosci, 26, 1548-1559.
Ashton, J.C. & Glass, M. (2007) The cannabinoid CB2 receptor as a target for inflammation-dependent
neurodegeneration. Curr Neuropharmacol, 5, 73-80.
Ashton, J.C., Appleton, I., Darlington, C.L. & Smith, P.F. (2004a) Immunohistochemical localization of
cannabinoid CB1 receptor in inhibitory interneurons in the cerebellum. Cerebellum, 3, 222-226.
Ashton, J.C., Friberg, D., Darlington, C.L. & Smith, P.F. (2006) Expression of the cannabinoid CB2 receptor in
the rat cerebellum: an immunohistochemical study. Neurosci Lett, 396, 113-116.
Ashton, J.C., Rahman, R.M., Nair, S.M., Sutherland, B.A., Glass, M. & Appleton, I. (2007) Cerebral hypoxia-
ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the
brain. Neurosci Lett, 412, 114-117.
Ashton, J.C., Zheng, Y., Liu, P., Darlington, C.L. & Smith, P.F. (2004b) Immunohistochemical characterisation
and localisation of cannabinoid CB1 receptor protein in the rat vestibular nucleus complex and the effects
of unilateral vestibular deafferentation. Brain Res, 1021, 264-271.
Astur, R.S., Taylor, L.B., Mamelak, A.N., Philpott, L. & Sutherland, R.J. (2002) Humans with hippocampus
damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res, 132,
77-84.
Bachtold, D., Baumann, T., Sandor, P.S., Kritos, M., Regard, M. & Brugger, P. (2001) Spatial- and verbal-
memory improvement by cold-water caloric stimulation in healthy subjects. Exp Brain Res, 136, 128-132.
Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2008) Cannabinoid CB2 receptor expression in the rat
brainstem cochlear and vestibular nuclei. Acta Otolaryngol, 128, 961-967.
Baek, J.H., Zheng, Y., Darlington, C.L. & Smith, P.F. (2009) The CB1 receptor agonist, WIN 55,212-2, dose-
dependently disrupts object recognition memory in adult rats. Neurosci Lett, 464, 71-73.
Bailey, C.H. & Kandel, E.R. (1993) Structural changes accompanying memory storage. Annu Rev Physiol, 55,
397-426.
Bajo, M., Roberto, M. & Schweitzer, P. (2009) Differential alteration of hippocampal excitatory synaptic
transmission by cannabinoid ligands. J Neurosci Res, 87, 766-775.
Baker, H.J., Lindsey, J. R. & Weisbroth, S. H. (ed) (1979) The Laboratory Rat. Academic Press, New York.
Baloh, R.W. (1979) Clinical neurophysiology of the vestibular system. F. A. Davis Co., Philadelphia.
Barmack, N.H. (2003) Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull, 60,
511-541.
Barna, I., Soproni, K., Arszovszki, A., Csabai, K. & Haller, J. (2007) WIN-55,212-2 chronically implanted into
the CA3 region of the dorsal hippocampus impairs learning: a novel method for studying chronic, brain-
area-specific effects of cannabinoids. Behav Pharmacol, 18, 515-520.
Barnes, C.A. (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in
the rat. J Comp Physiol Psychol, 93, 74-104.
Barnes, C.A. (1979) Memory deficits associated with senescence: a neurophysiological and behavioral study in

References 166
the rat. J Comp Physiol Psychol, 93, 74-104.
Barnes, C.A. (2004) Spatial memory loss of normal aging: Animal models and neural mechanisms. In Smelser,
N.J., Baltes, P. B. (ed) International Encyclopedia of the Social and Behavioural Sciences. Elsevier, pp.
14804-14807.
Bassett, J.P., Tullman, M.L. & Taube, J.S. (2007) Lesions of the tegmentomammillary circuit in the head
direction system disrupt the head direction signal in the anterior thalamus. J Neurosci, 27, 7564-7577.
Bastschelet, E. (1981) Circular statistics in biology. Academic, London.
Baude, A., Nusser, Z., Roberts, J.D., Mulvihill, E., McIlhinney, R.A. & Somogyi, P. (1993) The metabotropic
glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations
as detected by immunogold reaction. Neuron, 11, 771-787.
Beltramo, M. & Piomelli, D. (2000) Carrier-mediated transport and enzymatic hydrolysis of the endogenous
cannabinoid 2-arachidonylglycerol. Neuroreport, 11, 1231-1235.
Benito, C., Núñez, E., Tolon, R.M., Carrier, E.J., Rabano, A., Hillard, C.J. & Romero, J. (2003) Cannabinoid
CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated
glia in Alzheimer's disease brains. J Neurosci, 23, 11136-11141.
Benito, C., Romero, J.P., Tolon, R.M., Clemente, D., Docagne, F., Hillard, C.J., Guaza, C. & Romero, J. (2007)
Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell
subtypes in human multiple sclerosis. J Neurosci, 27, 2396-2402.
Bense, S., Deutschlander, A., Stephan, T., Bartenstein, P., Schwaiger, M., Brandt, T. & Dieterich, M. (2004)
Preserved visual-vestibular interaction in patients with bilateral vestibular failure. Neurology, 63, 122-128.
Beraneck, M., McKee, J.L., Aleisa, M. & Cullen, K.E. (2008) Asymmetric recovery in cerebellar-deficient mice
following unilateral labyrinthectomy. J Neurophysiol, 100, 945-958.
Bergman, J., Delatte, M.S., Paronis, C.A., Vemuri, K., Thakur, G.A. & Makriyannis, A. (2008) Some effects of
CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav, 93, 666-670.
Bergquist, F., Ludwig, M. & Dutia, M.B. (2008) Role of the commissural inhibitory system in vestibular
compensation in the rat. J Physiol, 586, 4441-4452.
Berrendero, F., Romero, J., Garcia-Gil, L., Suarez, I., De la Cruz, P., Ramos, J.A. & Fernandez-Ruiz, J.J. (1998)
Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim
Biophys Acta, 1407, 205-214.
Berrendero, F., Sanchez, A., Cabranes, A., Puerta, C., Ramos, J.A., Garcia-Merino, A. & Fernandez-Ruiz, J.
(2001) Changes in cannabinoid CB(1) receptors in striatal and cortical regions of rats with experimental
allergic encephalomyelitis, an animal model of multiple sclerosis. Synapse, 41, 195-202.
Bhatti, A.S., Aydin, C., Oztan, O., Ma, Z., Hall, P., Tao, R. & Isgor, C. (2009) Effects of a cannabinoid receptor
(CB) 1 antagonist AM251 on behavioral sensitization to nicotine in a rat model of novelty-seeking
behavior: correlation with hippocampal 5HT. Psychopharmacology (Berl), 203, 23-32.
Bisogno, T., MacCarrone, M., De Petrocellis, L., Jarrahian, A., Finazzi-Agro, A., Hillard, C. & Di Marzo, V.
(2001) The uptake by cells of 2-arachidonoylglycerol, an endogenous agonist of cannabinoid receptors.
Eur J Biochem, 268, 1982-1989.
Bisogno, T., Melck, D., Bobrov, M., Gretskaya, N.M., Bezuglov, V.V., De Petrocellis, L. & Di Marzo, V. (2000)
N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide

References 167
inactivation with cannabimimetic activity in vitro and in vivo. Biochem J, 351 Pt 3, 817-824.
Bisogno, T., Sepe, N., Melck, D., Maurelli, S., De Petrocellis, L. & Di Marzo, V. (1997) Biosynthesis, release
and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse
neuroblastoma cells. Biochem J, 322 ( Pt 2), 671-677.
Blanks, R.H., Curthoys, I.S. & Markham, C.H. (1972) Planar relationships of semicircular canals in the cat. Am J
Physiol, 223, 55-62.
Blanks, R.H., Curthoys, I.S. & Markham, C.H. (1975) Planar relationships of the semicircular canals in man.
Acta Otolaryngol, 80, 185-196.
Bockisch, C.J., Straumann, D., Hess, K. & Haslwanter, T. (2004) Enhanced smooth pursuit eye movements in
patients with bilateral vestibular deficits. Neuroreport, 15, 2617-2620.
Bohme, G.A., Laville, M., Ledent, C., Parmentier, M. & Imperato, A. (2000) Enhanced long-term potentiation in
mice lacking cannabinoid CB1 receptors. Neuroscience, 95, 5-7.
Bosier, B., Muccioli, G.G., Hermans, E. & Lambert, D.M. (2010) Functionally selective cannabinoid receptor
signalling: therapeutic implications and opportunities. Biochem Pharmacol, 80, 1-12.
Bouaboula, M., Poinot-Chazel, C., Bourrie, B., Canat, X., Calandra, B., Rinaldi-Carmona, M., Le Fur, G. &
Casellas, P. (1995) Activation of mitogen-activated protein kinases by stimulation of the central
cannabinoid receptor CB1. Biochem J, 312 ( Pt 2), 637-641.
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein
utilizing the principle of protein-dye binding. Anal Biochem, 72, 248-254.
Bradshaw, A.P., Curthoys, I.S., Todd, M.J., Magnussen, J.S., Taubman, D.S., Aw, S.T. & Halmagyi, G.M. A
mathematical model of human semicircular canal geometry: a new basis for interpreting vestibular
physiology. J Assoc Res Otolaryngol, 11, 145-159.
Brady, A.M., Saul, R.D. & Wiest, M.K. Selective deficits in spatial working memory in the neonatal ventral
hippocampal lesion rat model of schizophrenia. Neuropharmacology.
Brandt, T. (1996) Bilateral vestibulopathy revisited. Eur J Med Res, 1, 361-368.
Brandt, T., Schautzer, F., Hamilton, D.A., Bruning, R., Markowitsch, H.J., Kalla, R., Darlington, C., Smith, P. &
Strupp, M. (2005) Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans.
Brain, 128, 2732-2741.
Breivogel, C.S. & Childers, S.R. (1998) The functional neuroanatomy of brain cannabinoid receptors. Neurobiol
Dis, 5, 417-431.
Breivogel, C.S. & Sim-Selley, L.J. (2009) Basic neuroanatomy and neuropharmacology of cannabinoids. Int Rev
Psychiatry, 21, 113-121.
Breivogel, C.S., Sim, L.J. & Childers, S.R. (1997) Regional differences in cannabinoid receptor/G-protein
coupling in rat brain. J Pharmacol Exp Ther, 282, 1632-1642.
Brenowitz, S.D. & Regehr, W.G. (2003) Calcium dependence of retrograde inhibition by endocannabinoids at
synapses onto Purkinje cells. J Neurosci, 23, 6373-6384.
Broadbent, N.J., Gaskin, S., Squire, L.R. & Clark, R.E. (2010) Object recognition memory and the rodent
hippocampus. Learn Mem, 17, 5-11.
Broadbent, N.J., Squire, L.R. & Clark, R.E. (2004) Spatial memory, recognition memory, and the hippocampus.
Proc Natl Acad Sci U S A, 101, 14515-14520.

References 168
Brodal, A. & Pompeiano, O. (1957) The vestibular nuclei in cat. J Anat, 91, 438-454.
Brodal, A. (1974) Handbook of sensory physiology, vestibular system, morphology. Springer, Berlin, Heidelberg,
New York.
Brodal, A. (1981) Neurological anatomy. Oxford University Press, New York.
Brodkin, J. & Moerschbaecher, J.M. (1997) SR141716A antagonizes the disruptive effects of cannabinoid
ligands on learning in rats. J Pharmacol Exp Ther, 282, 1526-1532.
Bromberg, W. (1934) Marijuana intoxication. Am J Psychiatry, 91, 303-330.
Brookes, G.B., Gresty, M.A., Nakamura, T. & Metcalfe, T. (1993) Sensing and controlling rotational orientation
in normal subjects and patients with loss of labyrinthine function. Am J Otol, 14, 349-351.
Brusco, A., Tagliaferro, P., Saez, T. & Onaivi, E.S. (2008a) Postsynaptic localization of CB2 cannabinoid
receptors in the rat hippocampus. Synapse, 62, 944-949.
Brusco, A., Tagliaferro, P.A., Saez, T. & Onaivi, E.S. (2008b) Ultrastructural localization of neuronal brain CB2
cannabinoid receptors. Ann N Y Acad Sci, 1139, 450-457.
Buckley, M.J., Booth, M.C., Rolls, E.T. & Gaffan, D. (2001) Selective perceptual impairments after perirhinal
cortex ablation. J Neurosci, 21, 9824-9836.
Buffalo, E.A., Ramus, S.J., Clark, R.E., Teng, E., Squire, L.R. & Zola, S.M. (1999) Dissociation between the
effects of damage to perirhinal cortex and area TE. Learn Mem, 6, 572-599.
Burgess, N., Maguire, E.A. & O'Keefe, J. (2002) The human hippocampus and spatial and episodic memory.
Neuron, 35, 625-641.
Bussey, T.J., Saksida, L.M. & Murray, E.A. (2003) Impairments in visual discrimination after perirhinal cortex
lesions: testing 'declarative' vs. 'perceptual-mnemonic' views of perirhinal cortex function. Eur J Neurosci,
17, 649-660.
Buttner-Ennever, J.A. (1999) A review of otolith pathways to brainstem and cerebellum. Ann N Y Acad Sci, 871,
51-64.
Bylund, D.B. & Toews, M.L. (1993) Radioligand binding methods: practical guide and tips. Am J Physiol, 265,
L421-429.
Cabranes, A., Pryce, G., Baker, D. & Fernandez-Ruiz, J. (2006) Changes in CB1 receptors in motor-related brain
structures of chronic relapsing experimental allergic encephalomyelitis mice. Brain Res, 1107, 199-205.
Campolongo, P., Roozendaal, B., Trezza, V., Hauer, D., Schelling, G., McGaugh, J.L. & Cuomo, V. (2009)
Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable
glucocorticoid modulation of memory. Proc Natl Acad Sci U S A, 106, 4888-4893.
Canas, P.M., Duarte, J.M., Rodrigues, R.J., Kofalvi, A. & Cunha, R.A. (2009) Modification upon aging of the
density of presynaptic modulation systems in the hippocampus. Neurobiol Aging, 30, 1877-1884.
Caprioli, A., Ghirardi, O., Giuliani, A., Ramacci, M.T. & Angelucci, L. (1991) Spatial learning and memory in
the radial maze: a longitudinal study in rats from 4 to 25 months of age. Neurobiol Aging, 12, 605-607.
Caprioli, A., Ghirardi, O., Ramacci, M.T. & Angelucci, L. (1990) Age-dependent deficits in radial maze
performance in the rat: effect of chronic treatment with acetyl-L-carnitine. Prog Neuropsychopharmacol
Biol Psychiatry, 14, 359-369.
Carleton, S.C. & Carpenter, M.B. (1984) Distribution of primary vestibular fibers in the brainstem and
cerebellum of the monkey. Brain Res, 294, 281-298.

References 169
Carlini, E.A., Hamaoui, A., Bieniek, D. & Korte, F. (1970) Effects of (--) delta-9-trans-tetrahydrocannabinol and
a synthetic derivative on maze performance of rats. Pharmacology, 4, 359-368.
Carlisle, S.J., Marciano-Cabral, F., Staab, A., Ludwick, C. & Cabral, G.A. (2002) Differential expression of the
CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation.
Int Immunopharmacol, 2, 69-82.
Cartwright, B.A. & Collett, T. S. (1982) How honey-bees use landmarks to guide their return to a food source.
Nature, 295, 560-564.
Cartwright, B.A. & Collett, T. S. (1983) Landmark learning in bees: experiments and models. J Comp Physiol A,
151, 521-543.
Castelli, M.P., Paola Piras, A., D'Agostino, A., Pibiri, F., Perra, S., Gessa, G.L., Maccarrone, M. & Pistis, M.
(2007) Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the
cannabinoid agonist WIN 55,212-2. Eur J Pharmacol, 573, 11-19.
Caulfield, M.P. & Brown, D.A. (1992) Cannabinoid receptor agonists inhibit Ca current in NG108-15
neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol, 106, 231-232.
Centonze, D., Bari, M., Rossi, S., Prosperetti, C., Furlan, R., Fezza, F., De Chiara, V., Battistini, L., Bernardi, G.,
Bernardini, S., Martino, G. & Maccarrone, M. (2007) The endocannabinoid system is dysregulated in
multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain, 130, 2543-2553.
Centonze, D., Battistini, L. & Maccarrone, M. (2008) The endocannabinoid system in peripheral lymphocytes as
a mirror of neuroinflammatory diseases. Curr Pharm Des, 14, 2370-2342.
Cha, Y.M., White, A.M., Kuhn, C.M., Wilson, W.A. & Swartzwelder, H.S. (2006) Differential effects of delta9-
THC on learning in adolescent and adult rats. Pharmacol Biochem Behav, 83, 448-455.
Chait, L.D. & Perry, J.L. (1992) Factors influencing self-administration of, and subjective response to, placebo
marijuana. Behav Pharmacol, 3, 545-552.
Chambers, A.P., Koopmans, H.S., Pittman, Q.J. & Sharkey, K.A. (2006) AM 251 produces sustained reductions
in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J
Pharmacol, 147, 109-116.
Chambers, A.P., Sharkey, K.A. & Koopmans, H.S. (2004) Cannabinoid (CB)1 receptor antagonist, AM 251,
causes a sustained reduction of daily food intake in the rat. Physiol Behav, 82, 863-869.
Chan, P.K., Chan, S.C. & Yung, W.H. (1998) Presynaptic inhibition of GABAergic inputs to rat substantia nigra
pars reticulata neurones by a cannabinoid agonist. Neuroreport, 9, 671-675.
Chaperon, F. & Thiébot, M.H. (1999) Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol,
13, 243-281.
Chaperon, F., Soubrie, P., Puech, A. J. & Thiebot, M. (1998) Involvement of central cannabinoids (CB1)
receptors in the establishment of place conditioning in rats. Psychopharmacology (Berl), 135, 324-332.
Cheng, K. (1988) Some psychophysics of the pigeon's use of landmarks. J Comp Physiol A, 162, 815-826.
Cheng, K. (1989) The vector sum model of pigeon landmark use. J Exp Psychol Anim Behav Process, 15, 366-
375.
Cheng, Y. & Prusoff, W.H. (1973) Relationship between the inhibition constant (K1) and the concentration of
inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol, 22,
3099-3108.

References 170
Chevaleyre, V. & Castillo, P.E. (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of
endocannabinoids in regulating excitability. Neuron, 38, 461-472.
Chevaleyre, V., Heifets, B.D., Kaeser, P.S., Sudhof, T.C. & Castillo, P.E. (2007) Endocannabinoid-mediated
long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron, 54, 801-812.
Chevaleyre, V., Takahashi, K.A. & Castillo, P.E. (2006) Endocannabinoid-mediated synaptic plasticity in the
CNS. Annu Rev Neurosci, 29, 37-76.
Childers, S.R. & Breivogel, C.S. (1998) Cannabis and endogenous cannabinoid systems. Drug Alcohol Depend,
51, 173-187.
Chiu, C.Q. & Castillo, P.E. (2008) Input-specific plasticity at excitatory synapses mediated by endocannabinoids
in the dentate gyrus. Neuropharmacology, 54, 68-78.
Choi, K.D., Oh, S.Y., Kim, H.J., Koo, J.W., Cho, B.M. & Kim, J.S. (2007) Recovery of vestibular imbalances
after vestibular neuritis. Laryngoscope, 117, 1307-1312.
Ciccocioppo, R., Antonelli, L., Biondini, M., Perfumi, M., Pompei, P. & Massi, M. (2002) Memory impairment
following combined exposure to delta(9)-tetrahydrocannabinol and ethanol in rats. Eur J Pharmacol, 449,
245-252.
Clark, R.E., Zola, S.M. & Squire, L.R. (2000) Impaired recognition memory in rats after damage to the
hippocampus. J Neurosci, 20, 8853-8860.
Clarke, J.R., Rossato, J.I., Monteiro, S., Bevilaqua, L.R., Izquierdo, I. & Cammarota, M. (2008) Posttraining
activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object
recognition long-term memory. Neurobiol Learn Mem, 90, 374-381.
Cohen, H. & Keshner, E.A. (1989) Current concepts of the vestibular system reviewed: 2. Visual/vestibular
interaction and spatial orientation. Am J Occup Ther, 43, 331-338.
Collett, T.S. (1975) Visual spatial memory in a hover-fly. J Comp Physiol A, 100, 59-84.
Collett, T.S. (1987) The use of visual landmarks by gerbils: reaching a goal when landmarks are displaced. J
Comp Physiol A, 160, 109-113.
Collins, D.R., Pertwee, R.G. & Davies, S.N. (1994) The action of synthetic cannabinoids on the induction of
long-term potentiation in the rat hippocampal slice. Eur J Pharmacol, 259, R7-8.
Collins, D.R., Pertwee, R.G. & Davies, S.N. (1995) Prevention by the cannabinoid antagonist, SR141716A, of
cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. Br J Pharmacol,
115, 869-870.
Compton, D.R., Rice, K.C., De Costa, B.R., Razdan, R.K., Melvin, L.S., Johnson, M.R. & Martin, B.R. (1993)
Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J
Pharmacol Exp Ther, 265, 218-226.
Cristino, L., de Petrocellis, L., Pryce, G., Baker, D., Guglielmotti, V. & Di Marzo, V. (2006)
Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential
vanilloid type 1 receptors in the mouse brain. Neuroscience, 139, 1405-1415.
Cullen, K.E., Minor, L.B., Beraneck, M. & Sadeghi, S.G. (2009) Neural substrates underlying vestibular
compensation: contribution of peripheral versus central processing. J Vestib Res, 19, 171-182.
Curthoys, I.S., Blanks, R.H. & Markham, C.H. (1977) Semicircular canal functional anatomy in cat, guinea pig
and man. Acta Otolaryngol, 83, 258-265.

References 171
Curthoys, I.S., Curthoys, E.J., Blanks, R.H. & Markham, C.H. (1975) The orientation of the semicircular canals
in the guinea pig. Acta Otolaryngol, 80, 197-205.
Curthoys, I.S., Uzun-Coruhlu, H., Wong, C.C., Jones, A.S. & Bradshaw, A.P. (2009) The configuration and
attachment of the utricular and saccular maculae to the temporal bone. New evidence from
microtomography-CT studies of the membranous labyrinth. Ann N Y Acad Sci, 1164, 13-18.
Cuthbert, P.C., Gilchrist, D.P., Hicks, S.L., MacDougall, H.G. & Curthoys, I.S. (2000) Electrophysiological
evidence for vestibular activation of the guinea pig hippocampus. Neuroreport, 11, 1443-1447.
D'Ambra, T.E., Estep, K.G., Bell, M.R., Eissenstat, M.A., Josef, K.A., Ward, S.J., Haycock, D.A., Baizman, E.R.,
Casiano, F.M., Beglin, N.C. & et al. (1992) Conformationally restrained analogues of pravadoline:
nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J Med Chem,
35, 124-135.
Da Silva, G.E. & Takahashi, R.N. (2002) SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial
learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry, 26,
321-325.
Dalton, V.S., Wang, H. & Zavitsanou, K. (2009) HU210-induced downregulation in cannabinoid CB1 receptor
binding strongly correlates with body weight loss in the adult rat. Neurochem Res, 34, 1343-1353.
Daniel, H., Rancillac, A. & Crepel, F. (2004) Mechanisms underlying cannabinoid inhibition of presynaptic
Ca2+ influx at parallel fibre synapses of the rat cerebellum. J Physiol, 557, 159-174.
Darlington, C.L. & Smith, P.F. (2000) Molecular mechanisms of recovery from vestibular damage in mammals:
recent advances. Prog Neurobiol, 62, 313-325.
Darlington, C.L., Dutia, M.B. & Smith, P.F. (2002) The contribution of the intrinsic excitability of vestibular
nucleus neurons to recovery from vestibular damage. Eur J Neurosci, 15, 1719-1727.
Darmani, N.A. (2001) The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor
depressant actions of WIN 55, 212-2. Eur J Pharmacol, 430, 49-58.
Davies, S.N., Pertwee, R.G. & Riedel, G. (2002) Functions of cannabinoid receptors in the hippocampus.
Neuropharmacology, 42, 993-1007.
Day, T.A., Rakhshan, F., Deutsch, D.G. & Barker, E.L. (2001) Role of fatty acid amide hydrolase in the transport
of the endogenous cannabinoid anandamide. Mol Pharmacol, 59, 1369-1375.
de Lago, E., Fernandez-Ruiz, J., Ortega-Gutierrez, S., Cabranes, A., Pryce, G., Baker, D., Lopez-Rodriguez, M.
& Ramos, J.A. (2006) UCM707, an inhibitor of the anandamide uptake, behaves as a symptom control
agent in models of Huntington's disease and multiple sclerosis, but fails to delay/arrest the progression of
different motor-related disorders. Eur Neuropsychopharmacol, 16, 7-18.
de March, Z., Zuccato, C., Giampa, C., Patassini, S., Bari, M., Gasperi, V., De Ceballos, M.L., Bernardi, G.,
Maccarrone, M., Cattaneo, E. & Fusco, F.R. (2008) Cortical expression of brain derived neurotrophic
factor and type-1 cannabinoid receptor after striatal excitotoxic lesions. Neuroscience, 152, 734-740.
de Oliveira Alvares, L., de Oliveira, L.F., Camboim, C., Diehl, F., Genro, B.P., Lanziotti, V.B. & Quillfeldt, J.A.
(2005) Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance,
but not in the open field habituation task, in rats. Neurobiol Learn Mem, 83, 119-124.
de Oliveira Alvares, L., Genro, B.P., Diehl, F. & Quillfeldt, J.A. (2008) Differential role of the hippocampal
endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem, 90,

References 172
1-9.
de Oliveira Alvares, L., Genro, B.P., Vaz Breda, R., Pedroso, M.F., Da Costa, J.C. & Quillfeldt, J.A. (2006)
AM251, a selective antagonist of the CB1 receptor, inhibits the induction of long-term potentiation and
induces retrograde amnesia in rats. Brain Res, 1075, 60-67.
Deadwyler, S.A. & Hampson, R.E. (1997) The significance of neural ensemble codes during behavior and
cognition. Annu Rev Neurosci, 20, 217-244.
Deadwyler, S.A., Goonawardena, A.V. & Hampson, R.E. (2007) Short-term memory is modulated by the
spontaneous release of endocannabinoids: evidence from hippocampal population codes. Behav Pharmacol,
18, 571-580.
Deadwyler, S.A., Hampson, R.E., Mu, J., Whyte, A. & Childers, S. (1995) Cannabinoids modulate voltage
sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp
Ther, 273, 734-743.
Della Santina, C.C., Potyagaylo, V., Migliaccio, A.A., Minor, L.B. & Carey, J.P. (2005) Orientation of human
semicircular canals measured by three-dimensional multiplanar CT reconstruction. J Assoc Res
Otolaryngol, 6, 191-206.
Demuth, D. G. & Molleman, A. (2006) Cannabinoid signaling. Life Sci, 78, 549-563.
Dere, E., Huston, J.P. & De Souza Silva, M.A. (2007) The pharmacology, neuroanatomy and neurogenetics of
one-trial object recognition in rodents. Neurosci Biobehav Rev, 31, 673-704.
Derocq, J.M., Segui, M., Marchand, J., Le Fur, G. & Casellas, P. (1995) Cannabinoids enhance human B-cell
growth at low nanomolar concentrations. FEBS Lett, 369, 177-182.
Detre, S., Saclani Jotti, G. & Dowsett, M. (1995) A "quickscore" method for immunohistochemical
semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol, 48, 876-878.
Deutsch, D.G., Glaser, S.T., Howell, J.M., Kunz, J.S., Puffenbarger, R.A., Hillard, C.J. & Abumrad, N. (2001)
The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem,
276, 6967-6973.
Devane, W.A., Dysarz, F.A., 3rd, Johnson, M.R., Melvin, L.S. & Howlett, A.C. (1988) Determination and
characterization of a cannabinoid receptor in rat brain. Mol Pharmacol, 34, 605-613.
Devane, W.A., Hanus, L., Breuer, A., Pertwee, R.G., Stevenson, L.A., Griffin, G., Gibson, D., Mandelbaum, A.,
Etinger, A. & Mechoulam, R. (1992) Isolation and structure of a brain constituent that binds to the
cannabinoid receptor. Science, 258, 1946-1949.
Di Marzo, V., Fontana, A., Cadas, H., Schinelli, S., Cimino, G., Schwartz, J.C. & Piomelli, D. (1994) Formation
and inactivation of endogenous cannabinoid anandamide in central neurons. Nature, 372, 686-691.
Di Marzo, V., Hill, M.P., Bisogno, T., Crossman, A.R. & Brotchie, J.M. (2000) Enhanced levels of endogenous
cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of
Parkinson's disease. FASEB J, 14, 1432-1438.
Diana, M.A. & Marty, A. (2004) Endocannabinoid-mediated short-term synaptic plasticity: depolarization-
induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br
J Pharmacol, 142, 9-19.
Diana, M.A., Levenes, C., Mackie, K. & Marty, A. (2002) Short-term retrograde inhibition of GABAergic
synaptic currents in rat Purkinje cells is mediated by endogenous cannabinoids. J Neurosci, 22, 200-208.

References 173
Dieringer, N. (1995) 'Vestibular compensation': neural plasticity and its relations to functional recovery after
labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol, 46, 97-129.
Dieterich, M., Bauermann, T., Best, C., Stoeter, P. & Schlindwein, P. (2007) Evidence for cortical visual
substitution of chronic bilateral vestibular failure (an fMRI study). Brain, 130, 2108-2116.
Dieterich, M., Bense, S., Stephan, T., Yousry, T.A. & Brandt, T. (2003) fMRI signal increases and decreases in
cortical areas during small-field optokinetic stimulation and central fixation. Exp Brain Res, 148, 117-127.
Dowie, M.J., Bradshaw, H.B., Howard, M.L., Nicholson, L.F., Faull, R.L., Hannan, A.J. & Glass, M. (2009)
Altered CB1 receptor and endocannabinoid levels precede motor symptom onset in a transgenic mouse
model of Huntington's disease. Neuroscience, 163, 456-465.
Drai, D., Benjamini, Y. & Golani, I. (2000) Statistical discrimination of natural modes of motion in rat
exploratory behavior. J Neurosci Methods, 96, 119-131.
Drews, E., Schneider, M. & Koch, M. (2005) Effects of the cannabinoid receptor agonist WIN 55,212-2 on
operant behavior and locomotor activity in rats. Pharmacol Biochem Behav, 80, 145-150.
Dutia, M.B. (2010) Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head
Neck Surg, 18, 420-424.
Egashira, N., Mishima, K., Iwasaki, K. & Fujiwara, M. (2002) Intracerebral microinjections of delta 9-
tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats.
Brain Res, 952, 239-245.
Egerton, A., Allison, C., Brett, R.R. & Pratt, J.A. (2006) Cannabinoids and prefrontal cortical function: insights
from preclinical studies. Neurosci Biobehav Rev, 30, 680-695.
Egertova, M. & Elphick, M.R. (2000) Localisation of cannabinoid receptors in the rat brain using antibodies to
the intracellular C-terminal tail of CB. J Comp Neurol, 422, 159-171.
Egertova, M., Giang, D.K., Cravatt, B.F. & Elphick, M.R. (1998) A new perspective on cannabinoid signalling:
complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol
Sci, 265, 2081-2085.
Eilam, D. & Golani, I. (1989) Home base behavior of rats (Rattus norvegicus) exploring a novel environment.
Behav Brain Res, 34, 199-211.
Ellert-Miklaszewska, A., Grajkowska, W., Gabrusiewicz, K., Kaminska, B. & Konarska, L. (2007) Distinctive
pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res,
1137, 161-169.
Elphick, M.R. & Egertova, M. (2001) The neurobiology and evolution of cannabinoid signalling. Philos Trans R
Soc Lond B Biol Sci, 356, 381-408.
Ennaceur, A. & Delacour, J. (1988) A new one-trial test for neurobiological studies of memory in rats. 1:
Behavioral data. Behav Brain Res, 31, 47-59.
Etienne, A.S. (1980) The orientation of the golden hamster to its nest-site after the elimination of various sensory
cues. Experientia, 36, 1048-1050.
Etienne, A.S., Maurer, R., Saucy, F. & Teroni, E. (1986) Short-distance homing in the golden hamster after a
passive outward journey. Anim Behav, 34, 696-715.
Falenski, K.W., Blair, R.E., Sim-Selley, L.J., Martin, B.R. & DeLorenzo, R.J. (2007) Status epilepticus causes a
long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat

References 174
pilocarpine model of acquired epilepsy. Neuroscience, 146, 1232-1244.
Falenski, K.W., Carter, D.S., Harrison, A.J., Martin, B.R., Blair, R.E. & DeLorenzo, R.J. (2009) Temporal
characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-
induced status epilepticus. Brain Res, 1262, 64-72.
Fan, N., Yang, H., Zhang, J. & Chen, C. (2010) Reduced expression of glutamate receptors and phosphorylation
of CREB are responsible for in vivo Delta9-THC exposure-impaired hippocampal synaptic plasticity. J
Neurochem, 112, 691-702.
Faraji, J., Lehmann, H., Metz, G.A. & Sutherland, R.J. (2008) Rats with hippocampal lesion show impaired
learning and memory in the ziggurat task: a new task to evaluate spatial behavior. Behav Brain Res, 189,
17-31.
Fedrowitz, M., Lindemann, S., Loscher, W. & Gernert, M. (2003) Altered spontaneous discharge rate and pattern
of basal ganglia output neurons in the circling (ci2) rat mutant. Neuroscience, 118, 867-878.
Fegley, D., Kathuria, S., Mercier, R., Li, C., Goutopoulos, A., Makriyannis, A. & Piomelli, D. (2004)
Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the
hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A, 101, 8756-8761.
Ferbinteanu, J. & McDonald, R.J. (2001) Dorsal/ventral hippocampus, fornix, and conditioned place preference.
Hippocampus, 11, 187-200.
Ferbinteanu, J., Ray, C. & McDonald, R.J. (2003) Both dorsal and ventral hippocampus contribute to spatial
learning in Long-Evans rats. Neurosci Lett, 345, 131-135.
Fernandez-Ruiz, J. (2009) The endocannabinoid system as a target for the treatment of motor dysfunction. Br J
Pharmacol, 156, 1029-1040.
Fernández-Ruiz, J., Romero, J., Velasco, G., Tolon, R.M., Ramos, J.A. & Guzman, M. (2007) Cannabinoid CB2
receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci, 28, 39-45.
Fernandez, M.M., E. R. (1962) Inertial guidance engineering. Prentice Hall, Englewood Cliffs, New Jersey.
Ferrari, F., Ottani, A., Vivoli, R. & Giuliani, D. (1999) Learning impairment produced in rats by the cannabinoid
agonist HU 210 in a water-maze task. Pharmacol Biochem Behav, 64, 555-561.
Fetter, M., Diener, H.-C. & Dichgans, J. (1991) Recovery of postural control after an acute unilateral vestibular
lesion in humans. J Vestib Res, 1, 373-383.
Fetter, M., Heimberger, J., Black, R., Hermann, W., Sievering, F. & Dichgans, J. (1996) Otolith-semicircular
canal interaction during postrotatory nystagmus in humans. Exp Brain Res, 108, 463-472.
Foldy, C., Neu, A., Jones, M.V. & Soltesz, I. (2006) Presynaptic, activity-dependent modulation of cannabinoid
type 1 receptor-mediated inhibition of GABA release. J Neurosci, 26, 1465-1469.
Forwood, S.E., Winters, B.D. & Bussey, T.J. (2005) Hippocampal lesions that abolish spatial maze performance
spare object recognition memory at delays of up to 48 hours. Hippocampus, 15, 347-355.
Fowler, C.J. & Jacobsson, S.O. (2002) Cellular transport of anandamide, 2-arachidonoylglycerol and
palmitoylethanolamide--targets for drug development? Prostaglandins Leukot Essent Fatty Acids, 66, 193-
200.
Fowler, C.J., Rojo, M.L. & Rodriguez-Gaztelumendi, A. (2010) Modulation of the endocannabinoid system:
neuroprotection or neurotoxicity? Exp Neurol, 224, 37-47.
Fox, K.M., Sterling, R.C. & Van Bockstaele, E.J. (2009) Cannabinoids and novelty investigation: influence of

References 175
age and duration of exposure. Behav Brain Res, 196, 248-253.
Freund, T.F., Katona, I. & Piomelli, D. (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol
Rev, 83, 1017-1066.
Frick, K.M., Baxter, M.G., Markowska, A.L., Olton, D.S. & Price, D.L. (1995) Age-related spatial reference and
working memory deficits assessed in the water maze. Neurobiol Aging, 16, 149-160.
Frisk, V. & Milner, B. (1990) The role of the left hippocampal region in the acquisition and retention of story
content. Neuropsychologia, 28, 349-359.
Fukudome, Y., Ohno-Shosaku, T., Matsui, M., Omori, Y., Fukaya, M., Tsubokawa, H., Taketo, M.M., Watanabe,
M., Manabe, T. & Kano, M. (2004) Two distinct classes of muscarinic action on hippocampal inhibitory
synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through
endocannabinoid signalling. Eur J Neurosci, 19, 2682-2692.
Furman, J.M. & Jacob, R.G. (2001) A clinical taxonomy of dizziness and anxiety in the otoneurological setting. J
Anxiety Disord, 15, 9-26.
Fusco, F.R., Martorana, A., Giampa, C., De March, Z., Farini, D., D'Angelo, V., Sancesario, G. & Bernardi, G.
(2004) Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse,
53, 159-167.
Galiègue, S., Mary, S., Marchand, J., Dussossoy, D., Carriere, D., Carayon, P., Bouaboula, M., Shire, D., Le Fur,
G. & Casellas, P. (1995) Expression of central and peripheral cannabinoid receptors in human immune
tissues and leukocyte subpopulations. Eur J Biochem, 232, 54-61.
Gall, C.M., Hess, U.S. & Lynch, G. (1998) Mapping brain networks engaged by, and changed by, learning.
Neurobiol Learn Mem, 70, 14-36.
Galve-Roperh, I., Aguado, T., Palazuelos, J. & Guzman, M. (2007) The endocannabinoid system and
neurogenesis in health and disease. Neuroscientist, 13, 109-114.
Gaoni, Y. & Mechoulam, R. (1964) Isolation, structure and partial synthesis of an active constituent of hashish. J
Am Chem Soc, 86, 1646-1647.
Gatley, S.J., Lan, R., Volkow, N.D., Pappas, N., King, P., Wong, C.T., Gifford, A.N., Pyatt, B., Dewey, S.L. &
Makriyannis, A. (1998) Imaging the brain marijuana receptor: development of a radioligand that binds to
cannabinoid CB1 receptors in vivo. J Neurochem, 70, 417-423.
Gavrilov, V.V., Wiener, S.I. & Berthoz, A. (1995) Enhanced hippocampal theta EEG during whole body rotations
in awake restrained rats. Neurosci Lett, 197, 239-241.
Gavrilov, V.V., Wiener, S.I. & Berthoz, A. (1996) Whole-body rotations enhance hippocampal theta rhythmic
slow activity in awake rats passively transported on a mobile robot. Ann N Y Acad Sci, 781, 385-398.
Georgieva, T., Devanathan, S., Stropova, D., Park, C.K., Salamon, Z., Tollin, G., Hruby, V.J., Roeske, W.R.,
Yamamura, H.I. & Varga, E. (2008) Unique agonist-bound cannabinoid CB1 receptor conformations
indicate agonist specificity in signaling. Eur J Pharmacol, 581, 19-29.
Gerdeman, G.L. & Lovinger, D.M. (2003) Emerging roles for endocannabinoids in long-term synaptic plasticity.
Br J Pharmacol, 140, 781-789.
Gerdeman, G.L., Ronesi, J. & Lovinger, D.M. (2002) Postsynaptic endocannabinoid release is critical to long-
term depression in the striatum. Nat Neurosci, 5, 446-451.
Gessa, G.L., Mascia, M.S., Casu, M.A. & Carta, G. (1997) Inhibition of hippocampal acetylcholine release by

References 176
cannabinoids: reversal by SR 141716A. Eur J Pharmacol, 327, R1-2.
Gifford, A.N. & Ashby, C.R., Jr. (1996) Electrically evoked acetylcholine release from hippocampal slices is
inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid
antagonist, SR 141716A. J Pharmacol Exp Ther, 277, 1431-1436.
Gifford, A.N., Bruneus, M., Gatley, S.J. & Volkow, N.D. (2000) Cannabinoid receptor-mediated inhibition of
acetylcholine release from hippocampal and cortical synaptosomes. Br J Pharmacol, 131, 645-650.
Gilbert, P.E. & Kesner, R.P. (2002) Role of the rodent hippocampus in paired-associate learning involving
associations between a stimulus and a spatial location. Behav Neurosci, 116, 63-71.
Gilchrist, D.P., Curthoys, I.S., Cartwright, A.D., Burgess, A.M., Topple, A.N. & Halmagyi, M. (1998) High
acceleration impulsive rotations reveal severe long-term deficits of the horizontal vestibulo-ocular reflex in
the guinea pig. Exp Brain Res, 123, 242-254.
Gill, E.W., Paton, W.D. & Pertwee, R.G. (1970) Preliminary experiments on the chemistry and pharmacology of
cannabis. Nature, 228, 134-136.
Glaser, J.R. & Glaser, E.M. (2000) Stereology, morphometry, and mapping: the whole is greater than the sum of
its parts. J Chem Neuroanat, 20, 115-126.
Glaser, S.T., Abumrad, N.A., Fatade, F., Kaczocha, M., Studholme, K.M. & Deutsch, D.G. (2003) Evidence
against the presence of an anandamide transporter. Proc Natl Acad Sci U S A, 100, 4269-4274.
Glass, M. & Felder, C.C. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors
augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J
Neurosci, 17, 5327-5333.
Glass, M., Dragunow, M. & Faull, R.L. (1997) Cannabinoid receptors in the human brain: a detailed anatomical
and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience, 77,
299-318.
Glass, M., Dragunow, M. & Faull, R.L. (2000) The pattern of neurodegeneration in Huntington's disease: a
comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human
basal ganglia in Huntington's disease. Neuroscience, 97, 505-519.
Glass, M., Faull, R.L. & Dragunow, M. (1993) Loss of cannabinoid receptors in the substantia nigra in
Huntington's disease. Neuroscience, 56, 523-527.
Glover, J.C. (2009) Vestibular System. In Squire, L.R. (ed) Encyclopedia of Neuroscience. Academic Press, pp.
127-132.
Goble, T.J, Møller, A.R. & Thompson, L.T. (2009) Acute high-intensity sound exposure alters responses of place
cells in hippocampus. Hear Res, 253, 52-59.
Goddard, M., Zheng, Y., Darlington, C. L., Smith, P. F. (2008a) Locomotor and exploratory behaviour in the rat
following bilateral vestibular deafferentation. Behavioural Neuroscience, 122, 448-459.
Goddard, M., Zheng, Y., Darlington, C.L. & Smith, P.F. (2008b) Monoamine transporter and enzyme expression
in the medial temporal lobe and frontal cortex following chronic bilateral vestibular loss. Neurosci Lett,
437, 107-110.
Goddard, M., Zheng, Y., Darlington, C.L. & Smith, P.F. (2008c) Synaptic protein expression in the medial
temporal lobe and frontal cortex following chronic bilateral vestibular loss. Hippocampus, 18, 440-444.
Golani, I., Benjamini, Y. & Eilam, D. (1993) Stopping behavior: constraints on exploration in rats (Rattus

References 177
norvegicus). Behav Brain Res, 53, 21-33.
Goldberg, M.E. & Hudspeth, A. J. (2000) The vestibular system. In Kandel, E.R., Schwartz, J. H. & Jessell, T. M.
(ed) Principles of Neural Science. McGraw-Hill, pp. 801-805.
Goldstein, N.S., Hunter, S., Forbes, S., Odish, E. & Tehrani, M. (2007) Estrogen receptor antibody incubation
time and extent of immunoreactivity in invasive carcinoma of the breast: the importance of optimizing
antibody avidity. Appl Immunohistochem Mol Morphol, 15, 203-207.
Gong, J.P., Onaivi, E.S., Ishiguro, H., Liu, Q.R., Tagliaferro, P.A., Brusco, A. & Uhl, G.R. (2006) Cannabinoid
CB2 receptors: immunohistochemical localization in rat brain. Brain Res, 1071, 10-23.
Gonzalez, S., Cebeira, M. & Femandez-Ruiz, J. (2005) Cannabinoid tolerance and dependence: A review of
studies in laboratory animals. Pharmacol Biochem Behav, 81, 300-318.
Goodrich-Hunsaker, N.J., Livingstone, S.A., Skelton, R.W. & Hopkins, R.O. (2010) Spatial deficits in a virtual
water maze in amnesic participants with hippocampal damage. Hippocampus, 20, 481-491.
Goonawardena, A.V., Riedel, G. & Hampson, R.E. (2010) Cannabinoids alter spontaneous firing, bursting, and
cell synchrony of hippocampal principal cells. Hippocampus.
Goparaju, S.K., Ueda, N., Yamaguchi, H. & Yamamoto, S. (1998) Anandamide amidohydrolase reacting with 2-
arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett, 422, 69-73.
Gould, J.L. (1987) Landmark learning by honey-bees. J Comp Physiol A, 35, 26-34.
Griffin, G., Wray, E.J., Tao, Q., McAllister, S.D., Rorrer, W.K., Aung, M.M., Martin, B.R. & Abood, M.E. (1999)
Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for
cannabinoid CB2 receptor absence in the rat central nervous system. Eur J Pharmacol, 377, 117-125.
Grimm, R.J., Hemenway, W.G., Lebray, P.R. & Black, F.O. (1989) The perilymph fistula syndrome defined in
mild head trauma. Acta Otolaryngol Suppl, 464, 1-40.
Grimsey, N.L., Goodfellow, C.E., Scotter, E.L., Dowie, M.J., Glass, M. & Graham, E.S. (2008) Specific
detection of CB1 receptors; cannabinoid CB1 receptor antibodies are not all created equal! J Neurosci
Methods, 171, 78-86.
Grossman, G.E., Leigh, R.J., Abel, L.A., Lanska, D.J. & Thurston, S.E. (1988) Frequency and velocity of
rotational head perturbations during locomotion. Exp Brain Res, 70, 470-476.
Grossman, G.E., Leigh, R.J., Bruce, E.N., Huebner, W.P. & Lanska, D.J. (1989) Performance of the human
vestibuloocular reflex during locomotion. J Neurophysiol, 62, 264-272.
Grunfeld, Y. & Edery, H. (1969) Psychopharmacological activity of the active constituents of hashish and some
related cannabinoids. Psychopharmacologia, 14, 200-210.
Gullapalli, S., Amrutkar, D., Gupta, S., Kandadi, M.R., Kumar, H., Gandhi, M., Karande, V. & Narayanan, S.
(2010) Characterization of active and inactive states of CB1 receptor and the differential binding state
modulation by cannabinoid agonists, antagonists and inverse agonists. Neuropharmacology, 58, 1215-1219.
Hain, T.C. & Helminski, J. O. (2007) Vestibular rehabilitation. F. A. Davis Company, Philadelphia.
Haj-Dahmane, S. & Shen, R.Y. (2010) Regulation of plasticity of glutamate synapses by endocannabinoids and
the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol, 588, 2589-2604.
Hájos, N., Katona, I., Naiem, S.S., MacKie, K., Ledent, C., Mody, I. & Freund, T.F. (2000) Cannabinoids inhibit
hippocampal GABAergic transmission and network oscillations. Eur J Neurosci, 12, 3239-3249.
Hajos, N., Ledent, C. & Freund, T.F. (2001) Novel cannabinoid-sensitive receptor mediates inhibition of

References 178
glutamatergic synaptic transmission in the hippocampus. Neuroscience, 106, 1-4.
Hall, W. & Degenhardt, L. (2009) Adverse health effects of non-medical cannabis use. Lancet, 374, 1383-1391.
Hamann, K.F., Reber, A., Hess, B.J. & Dieringer, N. (1998) Long-term deficits in otolith, canal and optokinetic
ocular reflexes of pigmented rats after unilateral vestibular nerve section. Exp Brain Res, 118, 331-340.
Hampson, R.E. & Deadwyler, S.A. (2000) Cannabinoids reveal the necessity of hippocampal neural encoding for
short-term memory in rats. J Neurosci, 20, 8932-8942.
Hanes, D.A. & McCollum, G. (2006) Cognitive-vestibular interactions: a review of patient difficulties and
possible mechanisms. J Vestib Res, 16, 75-91.
Hanus, L., Abu-Lafi, S., Fride, E., Breuer, A., Vogel, Z., Shalev, D.E., Kustanovich, I. & Mechoulam, R. (2001)
2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci
U S A, 98, 3662-3665.
Harker, K.T. & Whishaw, I.Q. (2004) Impaired place navigation in place and matching-to-place swimming pool
tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus, 14, 224-
231.
He, J., Yamada, K. & Nabeshima, T. (2002) A role of Fos expression in the CA3 region of the hippocampus in
spatial memory formation in rats. Neuropsychopharmacology, 26, 259-268.
Heifets, B.D. & Castillo, P.E. (2009) Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev
Physiol, 71, 283-306.
Heimbrand, S., Muller, M., Schweigart, G. & Mergner, T. (1991) Perception of horizontal head and trunk
rotation in space in patients with loss of vestibular functions. J Vestib Res, 1, 291-298.
Heishman, S.J., Arasteh, K. & Stitzer, M.L. (1997) Comparative effects of alcohol and marijuana on mood,
memory, and performance. Pharmacol Biochem Behav, 58, 93-101.
Helmchen, C., Klinkenstein, J., Machner, B., Rambold, H., Mohr, C. & Sander, T. (2009) Structural changes in
the human brain following vestibular neuritis indicate central vestibular compensation. Ann N Y Acad Sci,
1164, 104-115.
Herdman, S.J., Hall, C.D., Schubert, M.C., Das, V.E. & Tusa, R.J. (2007) Recovery of dynamic visual acuity in
bilateral vestibular hypofunction. Arch Otolaryngol Head Neck Surg, 133, 383-389.
Herkenham, M. (1995) Cannabinoid receptors. Academic Press, London.
Herkenham, M., Lynn, A.B., Johnson, M.R., Melvin, L.S., de Costa, B.R. & Rice, K.C. (1991) Characterization
and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J
Neurosci, 11, 563-583.
Herkenham, M., Lynn, A.B., Little, M.D., Johnson, M.R., Melvin, L.S., de Costa, B.R. & Rice, K.C. (1990)
Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A, 87, 1932-1936.
Heskin-Sweezie, R., Titley, H.K., Baizer, J.S. & Broussard, D.M. (2010) Type B GABA receptors contribute to
the restoration of balance during vestibular compensation in mice. Neuroscience, 169, 302-314.
Hess, U.S., Lynch, G. & Gall, C.M. (1995) Changes in c-fos mRNA expression in rat brain during odor
discrimination learning: differential involvement of hippocampal subfields CA1 and CA3. J Neurosci, 15,
4786-4795.
Heyser, C.J., Hampson, R.E. & Deadwyler, S.A. (1993) Effects of delta-9-tetrahydrocannabinol on delayed
match to sample performance in rats: alterations in short-term memory associated with changes in task

References 179
specific firing of hippocampal cells. J Pharmacol Exp Ther, 264, 294-307.
Hicks, S. L., Gilchrist, D. P. D., Curthbert, P. & Curthoys, I. S. (2004) Hippocampal field responses to direct
electrical stimulation of the vestibular system in awake or anesthetised guinea pigs. Proc Aust Neurosci Soc,
15, 87.
Higgs, S., Barber, D.J., Cooper, A.J. & Terry, P. (2005) Differential effects of two cannabinoid receptor agonists
on progressive ratio responding for food and free-feeding in rats. Behav Pharmacol, 16, 389-393.
Highstein, S.M. & Holstein, G.R. (2006) The anatomy of the vestibular nuclei. Prog Brain Res, 151, 157-203.
Hill, A.J. & Best, P.J. (1981) Effects of deafness and blindness on the spatial correlates of hippocampal unit
activity in the rat. Exp Neurol, 74, 204-217.
Hill, M.N., Carrier, E.J., Ho, W.S., Shi, L., Patel, S., Gorzalka, B.B. & Hillard, C.J. (2008a) Prolonged
glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus,
18, 221-226.
Hill, M.N., Carrier, E.J., McLaughlin, R.J., Morrish, A.C., Meier, S.E., Hillard, C.J. & Gorzalka, B.B. (2008b)
Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent
antidepressant treatment. J Neurochem, 106, 2322-2336.
Hill, M.N., Froc, D.J., Fox, C.J., Gorzalka, B.B. & Christie, B.R. (2004) Prolonged cannabinoid treatment results
in spatial working memory deficits and impaired long-term potentiation in the CA1 region of the
hippocampus in vivo. Eur J Neurosci, 20, 859-863.
Hill, M.N., Hunter, R.G. & McEwen, B.S. (2009) Chronic stress differentially regulates cannabinoid CB1
receptor binding in distinct hippocampal subfields. Eur J Pharmacol, 614, 66-69.
Hill, M.N., Patel, S., Carrier, E.J., Rademacher, D.J., Ormerod, B.K., Hillard, C.J. & Gorzalka, B.B. (2005)
Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress.
Neuropsychopharmacology, 30, 508-515.
Hill, M.N., Titterness, A.K., Morrish, A.C., Carrier, E.J., Lee, T.T., Gil-Mohapel, J., Gorzalka, B.B., Hillard, C.J.
& Christie, B.R. (2010) Endogenous cannabinoid signaling is required for voluntary exercise-induced
enhancement of progenitor cell proliferation in the hippocampus. Hippocampus, 20, 513-523.
Hillard, C.J. & Jarrahian, A. (2000) The movement of N-arachidonoylethanolamine (anandamide) across cellular
membranes. Chem Phys Lipids, 108, 123-134.
Hillard, C.J., Edgemond, W.S. & Campbell, W.B. (1995) Characterization of ligand binding to the cannabinoid
receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem, 64, 677-
683.
Hillard, C.J., Manna, S., Greenberg, M.J., DiCamelli, R., Ross, R.A., Stevenson, L.A., Murphy, V., Pertwee, R.G.
& Campbell, W.B. (1999) Synthesis and characterization of potent and selective agonists of the neuronal
cannabinoid receptor (CB1). J Pharmacol Exp Ther, 289, 1427-1433.
Him, A. & Dutia, M.B. (2001) Intrinsic excitability changes in vestibular nucleus neurons after unilateral
deafferentation. Brain Res, 908, 58-66.
Hines, D.J. & Whishaw, I.Q. (2005) Home bases formed to visual cues but not to self-movement (dead
reckoning) cues in exploring hippocampectomized rats. Eur J Neurosci, 22, 2363-2375.
Hodos, W. (1961) Progressive ratio as a measure of reward strength. Science, 134, 943-944.
Hoffman, A.F. & Lupica, C.R. (2000) Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission

References 180
in the hippocampus. J Neurosci, 20, 2470-2479.
Hoffman, A.F., Oz, M., Yang, R., Lichtman, A.H. & Lupica, C.R. (2007) Opposing actions of chronic Delta9-
tetrahydrocannabinol and cannabinoid antagonists on hippocampal long-term potentiation. Learn Mem, 14,
63-74.
Holahan, M.R. & Routtenberg, A. (2010) Lidocaine injections targeting CA3 hippocampus impair long-term
spatial memory and prevent learning-induced mossy fiber remodeling. Hippocampus.
Hollister, L.E. (1974) Structure-activity relationships in man of cannabis constituents, and homologs and
metabolites of delta9-tetrahydrocannabinol. Pharmacology, 11, 3-11.
Honrubia, V., Jenkins, H.A., Baloh, R.W., Yee, R.D. & Lau, C.G. (1984) Vestibulo-ocular reflexes in peripheral
labyrinthine lesions: I. Unilateral dysfunction. Am J Otolaryngol, 5, 15-26.
Honrubia, V., Marco, J., Andrews, J., Minser, K., Yee, R.D. & Baloh, R.W. (1985) Vestibulo-ocular reflexes in
peripheral labyrinthine lesions: III. Bilateral dysfunction. Am J Otolaryngol, 6, 342-352.
Horak, F.B. (2009) Postural compensation for vestibular loss. Ann N Y Acad Sci, 1164, 76-81.
Horak, F.B., Nashner, L.M. & Diener, H.C. (1990) Postural strategies associated with somatosensory and
vestibular loss. Exp Brain Res, 82, 167-177.
Horii, A., Kitahara, T., Smith, P.F., Darlington, C.L., Masumura, C. & Kubo, T. (2003) Effects of unilateral
labyrinthectomy on GAD, GAT1 and GABA receptor gene expression in the rat vestibular nucleus.
Neuroreport, 14, 2359-2363.
Horii, A., Russell, N.A., Smith, P.F., Darlington, C.L. & Bilkey, D.K. (2004) Vestibular influences on CA1
neurons in the rat hippocampus: an electrophysiological study in vivo. Exp Brain Res, 155, 245-250.
Horii, A., Takeda, N., Mochizuki, T., Okakura-Mochizuki, K., Yamamoto, Y. & Yamatodani, A. (1994) Effects of
vestibular stimulation on acetylcholine release from rat hippocampus: an in vivo microdialysis study. J
Neurophysiol, 72, 605-611.
Horii, A., Takeda, N., Mochizuki, T., Okakura-Mochizuki, K., Yamamoto, Y., Yamatodani, A. & Kubo, T. (1995)
Vestibular modulation of the septo-hippocampal cholinergic system of rats. Acta Otolaryngol Suppl, 520 Pt
2, 395-398.
Horii, A., Takeda, N., Yamatodani, A. & Kubo, A.T. (1996) Vestibular influences on the histaminergic and
cholinergic systems in the rat brain. Ann N Y Acad Sci, 781, 633-634.
Hosseini-Sharifabad, M. & Nyengaard, J.R. (2007) Design-based estimation of neuronal number and individual
neuronal volume in the rat hippocampus. J Neurosci Methods, 162, 206-214.
Houston, D.B. & Howlett, A.C. (1993) Solubilization of the cannabinoid receptor from rat brain and its
functional interaction with guanine nucleotide-binding proteins. Mol Pharmacol, 43, 17-22.
Houston, D.B. & Howlett, A.C. (1998) Differential receptor-G-protein coupling evoked by dissimilar
cannabinoid receptor agonists. Cell Signal, 10, 667-674.
Howlett, A.C. & Fleming, R.M. (1984) Cannabinoid inhibition of adenylate cyclase. Pharmacology of the
response in neuroblastoma cell membranes. Mol Pharmacol, 26, 532-538.
Howlett, A.C. (1984) Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds.
Life Sci, 35, 1803-1810.
Howlett, A.C. (1985) Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in
neuroblastoma cell membranes. Mol Pharmacol, 27, 429-436.

References 181
Howlett, A.C. (2002) The cannabinoid receptors. Prostaglandins Other Lipid Mediat, 68-69, 619-631.
Howlett, A.C. (2005) Cannabinoid receptor signaling. Handb Exp Pharmacol, 53-79.
Howlett, A.C., Barth, F., Bonner, T.I., Cabral, G., Casellas, P., Devane, W.A., Felder, C.C., Herkenham, M.,
Mackie, K., Martin, B.R., Mechoulam, R. & Pertwee, R.G. (2002) International Union of Pharmacology.
XXVII. Classification of cannabinoid receptors. Pharmacol Rev, 54, 161-202.
Howlett, A.C., Qualy, J.M. & Khachatrian, L.L. (1986) Involvement of Gi in the inhibition of adenylate cyclase
by cannabimimetic drugs. Mol Pharmacol, 29, 307-313.
Huang, S.M., Bisogno, T., Trevisani, M., Al-Hayani, A., De Petrocellis, L., Fezza, F., Tognetto, M., Petros, T.J.,
Krey, J.F., Chu, C.J., Miller, J.D., Davies, S.N., Geppetti, P., Walker, J.M. & Di Marzo, V. (2002) An
endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors.
Proc Natl Acad Sci U S A, 99, 8400-8405.
Hüfner, K., Binetti, C., Hamilton, D.A., Stephan, T., Flanagin, V.L., Linn, J., Labudda, K., Markowitsch, H.,
Glasauer, S., Jahn, K., Strupp, M. & Brandt, T. (2010) Structural and functional plasticity of the
hippocampal formation in professional dancers and slackliners. Hippocampus.
Hüfner, K., Hamilton, D.A., Kalla, R., Stephan, T., Glasauer, S., Ma, J., Bruning, R., Markowitsch, H.J.,
Labudda, K., Schichor, C., Strupp, M. & Brandt, T. (2007) Spatial memory and hippocampal volume in
humans with unilateral vestibular deafferentation. Hippocampus, 17, 471-485.
Hüfner, K., Stephan, T., Hamilton, D.A., Kalla, R., Glasauer, S., Strupp, M. & Brandt, T. (2009) Gray-matter
atrophy after chronic complete unilateral vestibular deafferentation. Ann N Y Acad Sci, 1164, 383-385.
Igarashi, M. & Guitierrez, O. (1983) Analysis of righting reflex in cats with unilateral and bilateral
labyrinthectomy. ORL J Otorhinolaryngol Relat Spec, 45, 279-289.
Igarashi, M., Ishikawa, K., Ishii, M. & Yamane, H. (1988) Physical exercise and balance compensation after total
ablation of vestibular organs. Prog Brain Res, 76, 395-401.
Inada, H., Maejima, T., Nakahata, Y., Yamaguchi, J., Nabekura, J. & Ishibashi, H. (2010) Endocannabinoids
contribute to metabotropic glutamate receptor-mediated inhibition of GABA release onto hippocampal
CA3 pyramidal neurons in an isolated neuron/bouton preparation. Neuroscience, 165, 1377-1389.
Iordanova, M.D., Burnett, D.J., Aggleton, J.P., Good, M. & Honey, R.C. (2009) The role of the hippocampus in
mnemonic integration and retrieval: complementary evidence from lesion and inactivation studies. Eur J
Neurosci, 30, 2177-2189.
Israel, I., Bronstein, A.M., Kanayama, R., Faldon, M. & Gresty, M.A. (1996) Visual and vestibular factors
influencing vestibular "navigation". Exp Brain Res, 112, 411-419.
Jansen, E.M., Haycock, D.A., Ward, S.J. & Seybold, V.S. (1992) Distribution of cannabinoid receptors in rat
brain determined with aminoalkylindoles. Brain Res, 575, 93-102.
Järbe, T.U., Ross, T., DiPatrizio, N.V., Pandarinathan, L. & Makriyannis, A. (2006) Effects of the CB1R agonist
WIN-55,212-2 and the CB1R antagonists SR-141716 and AM-1387: open-field examination in rats.
Pharmacol Biochem Behav, 85, 243-252.
Jarrard, L.E. (1993) On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol, 60, 9-
26.
Jauregui-Renaud, K., Sang, F.Y., Gresty, M.A., Green, D.A. & Bronstein, A.M. (2008)
Depersonalisation/derealisation symptoms and updating orientation in patients with vestibular disease. J

References 182
Neurol Neurosurg Psychiatry, 79, 276-283.
Jelsing, J., Larsen, P.J. & Vrang, N. (2008) Identification of cannabinoid type 1 receptor expressing cocaine
amphetamine-regulated transcript neurons in the rat hypothalamus and brainstem using in situ
hybridization and immunohistochemistry. Neuroscience, 154, 641-652.
Jensen, D.W. (1983) Survival of function in the deafferentated vestibular nerve. Brain Res, 273, 175-178.
Jhaveri, M.D., Elmes, S.J., Richardson, D., Barrett, D.A., Kendall, D.A., Mason, R. & Chapman, V. (2008)
Evidence for a novel functional role of cannabinoid CB(2) receptors in the thalamus of neuropathic rats.
Eur J Neurosci, 27, 1722-1730.
Jin, K., Xie, L., Kim, S.H., Parmentier-Batteur, S., Sun, Y., Mao, X.O., Childs, J. & Greenberg, D.A. (2004)
Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol, 66, 204-208.
Jones, G., Pertwee, R.G., Gill, E.W., Paton, W.D., Nilsson, I.M., Widman, M. & Agurell, S. (1974) Relative
pharmacological potency in mice of optical isomers of delta 1-tetrahydrocannabinol. Biochem Pharmacol,
23, 439-446.
Jones, R. (1977) Human effects. NIDA Res Monogr, 128-178.
Kaiser, A., Fedrowitz, M., Ebert, U., Zimmermann, E., Hedrich, H.J., Wedekind, D. & Loscher, W. (2001)
Auditory and vestibular defects in the circling (ci2) rat mutant. Eur J Neurosci, 14, 1129-1142.
Kalueff, A.V., Ishikawa, K. & Griffith, A.J. (2008) Anxiety and otovestibular disorders: linking behavioral
phenotypes in men and mice. Behav Brain Res, 186, 1-11.
Kaminski, N.E., Abood, M.E., Kessler, F.K., Martin, B.R. & Schatz, A.R. (1992) Identification of a functionally
relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune
modulation. Mol Pharmacol, 42, 736-742.
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M. & Watanabe, M. (2009) Endocannabinoid-
mediated control of synaptic transmission. Physiol Rev, 89, 309-380.
Karnath, H.O. & Dieterich, M. (2006) Spatial neglect--a vestibular disorder? Brain, 129, 293-305.
Kathmann, M., Bauer, U., Schlicker, E. & Gothert, M. (1999) Cannabinoid CB1 receptor-mediated inhibition of
NMDA- and kainate-stimulated noradrenaline and dopamine release in the brain. Naunyn Schmiedebergs
Arch Pharmacol, 359, 466-470.
Katona, I., Rancz, E.A., Acsady, L., Ledent, C., Mackie, K., Hajos, N. & Freund, T.F. (2001) Distribution of CB1
cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci,
21, 9506-9518.
Katona, I., Sperlagh, B., Magloczky, Z., Santha, E., Kofalvi, A., Czirjak, S., Mackie, K., Vizi, E.S. & Freund, T.F.
(2000) GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus.
Neuroscience, 100, 797-804.
Katona, I., Sperlagh, B., Sik, A., Kafalvi, A., Vizi, E.S., Mackie, K. & Freund, T.F. (1999) Presynaptically
located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal
interneurons. J Neurosci, 19, 4544-4558.
Katona, I., Urban, G.M., Wallace, M., Ledent, C., Jung, K.M., Piomelli, D., Mackie, K. & Freund, T.F. (2006)
Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci, 26, 5628-
5637.
Kawamura, Y., Fukaya, M., Maejima, T., Yoshida, T., Miura, E., Watanabe, M., Ohno-Shosaku, T. & Kano, M.

References 183
(2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in
the hippocampus and cerebellum. J Neurosci, 26, 2991-3001.
Kearn, C.S., Greenberg, M.J., DiCamelli, R., Kurzawa, K. & Hillard, C.J. (1999) Relationships between ligand
affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J
Neurochem, 72, 2379-2387.
Keshner, E.A. & Cohen, H. (1989) Current concepts of the vestibular system reviewed: 1. The role of the
vestibulospinal system in postural control. Am J Occup Ther, 43, 320-330.
Kesner, R.P. (2007) Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem, 14, 771-781.
Kessels, R.P., de Haan, E.H., Kappelle, L.J. & Postma, A. (2001) Varieties of human spatial memory: a meta-
analysis on the effects of hippocampal lesions. Brain Res Brain Res Rev, 35, 295-303.
Keuker, J.I., Vollmann-Honsdorf, G.K. & Fuchs, E. (2001) How to use the optical fractionator: an example based
on the estimation of neurons in the hippocampal CA1 and CA3 regions of tree shrews. Brain Res Brain Res
Protoc, 7, 211-221.
Kim, J. & Alger, B.E. (2010) Reduction in endocannabinoid tone is a homeostatic mechanism for specific
inhibitory synapses. Nat Neurosci, 13, 592-600.
Kim, J., Isokawa, M., Ledent, C. & Alger, B.E. (2002) Activation of muscarinic acetylcholine receptors enhances
the release of endogenous cannabinoids in the hippocampus. J Neurosci, 22, 10182-10191.
Kim, M.S., Jin, B.K., Chun, S.W., Lee, M.Y., Lee, S.H., Kim, J.H. & Park, B.R. (1997) Effect of MK801 on
cFos-like protein expression in the medial vestibular nucleus at early stage of vestibular compensation in
uvulonodullectomized rats. Neurosci Lett, 231, 147-150.
King, J., Zheng, Y., Liu, P., Darlington, C.L. & Smith, P.F. (2002) NMDA and AMPA receptor subunit protein
expression in the rat vestibular nucleus following unilateral labyrinthectomy. Neuroreport, 13, 1541-1545.
Klein, T.W., Newton, C.A. & Friedman, H. (2001) Cannabinoids and the immune system. Pain Res Manag, 6,
95-101.
Knierim, J.J., Kudrimoti, H.S. & McNaughton, B.L. (1995) Place cells, head direction cells, and the learning of
landmark stability. J Neurosci, 15, 1648-1659.
Koethe, D., Llenos, I.C., Dulay, J.R., Hoyer, C., Torrey, E.F., Leweke, F.M. & Weis, S. (2007) Expression of
CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major
depression. J Neural Transm, 114, 1055-1063.
Kondrachuk, A.V. (2001) Simulation and interpretation of experiments with otoliths. J Gravit Physiol, 8, P101-
104.
Kosiorek, P., Hryniewicz, A., Bialuk, I., Zawadzka, A. & Winnicka, M.M. (2003) Cannabinoids alter recognition
memory in rats. Pol J Pharmacol, 55, 903-910.
Kreitzer, A.C. & Regehr, W.G. (2001a) Cerebellar depolarization-induced suppression of inhibition is mediated
by endogenous cannabinoids. J Neurosci, 21, RC174.
Kreitzer, A.C. & Regehr, W.G. (2001b) Retrograde inhibition of presynaptic calcium influx by endogenous
cannabinoids at excitatory synapses onto Purkinje cells. Neuron, 29, 717-727.
Kubie, J.L., Muller, R.U. & Bostock, E. (1990) Spatial firing properties of hippocampal theta cells. J Neurosci,
10, 1110-1123.
Kutner, M.H., Nachtsheim, C.J., Neter, J. & Li, W. (2005) Applied Linear Statistical Models. McGraw-Hill Irwin,

References 184
Boston.
Lacour, M. & Tighilet, B. (2010) Plastic events in the vestibular nuclei during vestibular compensation: the brain
orchestration of a "deafferentation" code. Restor Neurol Neurosci, 28, 19-35.
Lafourcade, C.A. (2009) Presynaptic mechanisms of endocannabinoid-mediated long-term depression in the
hippocampus. J Neurophysiol, 102, 2009-2012.
Landsman, R.S., Burkey, T.H., Consroe, P., Roeske, W.R. & Yamamura, H.I. (1997) SR141716A is an inverse
agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol, 334, R1-2.
Lane, J.I., Witte, R.J., Bolster, B., Bernstein, M.A., Johnson, K. & Morris, J. (2008) State of the art: 3T imaging
of the membranous labyrinth. AJNR Am J Neuroradiol, 29, 1436-1440.
Lawston, J., Borella, A., Robinson, J.K. & Whitaker-Azmitia, P.M. (2000) Changes in hippocampal morphology
following chronic treatment with the synthetic cannabinoid WIN 55,212-2. Brain Res, 877, 407-410.
Lecuit, T. & Lenne, P.F. (2007) Cell surface mechanics and the control of cell shape, tissue patterns and
morphogenesis. Nat Rev Mol Cell Biol, 8, 633-644.
Ledent, C., Valverde, O., Cossu, G., Petitet, F., Aubert, J.F., Beslot, F., Bohme, G.A., Imperato, A., Pedrazzini, T.,
Roques, B.P., Vassart, G., Fratta, W. & Parmentier, M. (1999) Unresponsiveness to cannabinoids and
reduced addictive effects of opiates in CB1 receptor knockout mice. Science, 283, 401-404.
Lee, S.H., Foldy, C. & Soltesz, I. (2010) Distinct endocannabinoid control of GABA release at perisomatic and
dendritic synapses in the hippocampus. J Neurosci, 30, 7993-8000.
Leenaars, M. & Hendriksen, C.F. (2005) Critical steps in the production of polyclonal and monoclonal antibodies:
evaluation and recommendations. ILAR J, 46, 269-279.
Lees, G. & Dougalis, A. (2004) Differential effects of the sleep-inducing lipid oleamide and cannabinoids on the
induction of long-term potentiation in the CA1 neurons of the rat hippocampus in vitro. Brain Res, 997, 1-
14.
Levenes, C., Daniel, H., Soubrie, P. & Crepel, F. (1998) Cannabinoids decrease excitatory synaptic transmission
and impair long-term depression in rat cerebellar Purkinje cells. J Physiol, 510 ( Pt 3), 867-879.
Li, H., Godfrey, D.A. & Rubin, A.M. (1997) Quantitative autoradiography of 5-[3H]6-cyano-7-nitro-
quinoxaline-2,3-dione and (+)-3-[3H]dizocilpine maleate binding in rat vestibular nuclear complex after
unilateral deafferentation, with comparison to cochlear nucleus. Neuroscience, 77, 473-484.
Li, H., Godfrey, T.G., Godfrey, D.A. & Rubin, A.M. (1996) Immunohistochemical study on the distributions of
AMPA receptor subtypes in rat vestibular nuclear complex after unilateral deafferentation. Ann N Y Acad
Sci, 781, 653-655.
Lichtman, A.H. & Martin, B.R. (1996) Delta 9-tetrahydrocannabinol impairs spatial memory through a
cannabinoid receptor mechanism. Psychopharmacology (Berl), 126, 125-131.
Lichtman, A.H. (2000) SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur
J Pharmacol, 404, 175-179.
Lichtman, A.H., Dimen, K.R. & Martin, B.R. (1995) Systemic or intrahippocampal cannabinoid administration
impairs spatial memory in rats. Psychopharmacology (Berl), 119, 282-290.
Lichtman, A.H., Varvel, S.A. & Martin, B.R. (2002) Endocannabinoids in cognition and dependence.
Prostaglandins Leukot Essent Fatty Acids, 66, 269-285.
Ligresti, A., Morera, E., Van Der Stelt, M., Monory, K., Lutz, B., Ortar, G. & Di Marzo, V. (2004) Further

References 185
evidence for the existence of a specific process for the membrane transport of anandamide. Biochem J, 380,
265-272.
Lindemann, S., Lessenich, A., Ebert, U. & Loscher, W. (2001) Spontaneous paroxysmal circling behavior in the
ci2 rat mutant: epilepsy with rotational seizures or hyperkinetic movement disorder? Exp Neurol, 172, 437-
445.
Lindsay, L., Liu, P., Gliddon, C., Zheng, Y., Smith, P.F. & Darlington, C.L. (2005) Cytosolic glucocorticoid
receptor expression in the rat vestibular nucleus and hippocampus following unilateral vestibular
deafferentation. Exp Brain Res, 162, 309-314.
Little, P.J., Compton, D.R., Johnson, M.R., Melvin, L.S. & Martin, B.R. (1988) Pharmacology and
stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther, 247, 1046-1051.
Liu, P., Bilkey, D.K., Darlington, C.L. & Smith, P.F. (2003a) Cannabinoid CB1 receptor protein expression in the
rat hippocampus and entorhinal, perirhinal, postrhinal and temporal cortices: regional variations and age-
related changes. Brain Res, 979, 235-239.
Liu, P., Gliddon, C.M., Lindsay, L., Darlington, C.L. & Smith, P.F. (2004) Nitric oxide synthase and arginase
expression changes in the rat perirhinal and entorhinal cortices following unilateral vestibular damage: a
link to deficits in object recognition? J Vestib Res, 14, 411-417.
Liu, P., Zheng, Y., King, J., Darlington, C.L. & Smith, P.F. (2003b) Long-term changes in hippocampal n-methyl-
D-aspartate receptor subunits following unilateral vestibular damage in rat. Neuroscience, 117, 965-970.
Liu, P., Zheng, Y., King, J., Darlington, C.L. & Smith, P.F. (2003c) Nitric oxide synthase and arginase expression
in the vestibular nucleus and hippocampus following unilateral vestibular deafferentation in the rat. Brain
Res, 966, 19-25.
Llano, I., Leresche, N. & Marty, A. (1991) Calcium entry increases the sensitivity of cerebellar Purkinje cells to
applied GABA and decreases inhibitory synaptic currents. Neuron, 6, 565-574.
Loewenstein, O.E. (1974) Comparative morphology and physiology. In Kornhuber, H.H. (ed) Handbook of
sensory physiology. Springer-Verlag, New York, pp. 75-122.
Lombard, C., Nagarkatti, M. & Nagarkatti, P. (2007) CB2 cannabinoid receptor agonist, JWH-015, triggers
apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin
Immunol, 122, 259-270.
Lopez, C., Lacour, M., Ahmadi, A.E., Magnan, J. & Borel, L. (2007) Changes of visual vertical perception: a
long-term sign of unilateral and bilateral vestibular loss. Neuropsychologia, 45, 2025-2037.
Lorincz, A. & Nusser, Z. (2008) Specificity of immunoreactions: the importance of testing specificity in each
method. J Neurosci, 28, 9083-9086.
Ludanyi, A., Eross, L., Czirjak, S., Vajda, J., Halasz, P., Watanabe, M., Palkovits, M., Magloczky, Z., Freund, T.F.
& Katona, I. (2008) Downregulation of the CB1 cannabinoid receptor and related molecular elements of
the endocannabinoid system in epileptic human hippocampus. J Neurosci, 28, 2976-2990.
Lutz, B. & Marsicano, G. (2009) Endocannabinoid role in synaptic plasticity and learning. In Squire, L.R. (ed)
Encyclopedia of Neuroscience. Academic Press, pp. 963-975.
Lynn, A.B. & Herkenham, M. (1994) Localization of cannabinoid receptors and nonsaturable high-density
cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune
modulation by cannabinoids. J Pharmacol Exp Ther, 268, 1612-1623.

References 186
Maaswinkel, H. & Whishaw, I.Q. (1999) Homing with locale, taxon, and dead reckoning strategies by foraging
rats: sensory hierarchy in spatial navigation. Behav Brain Res, 99, 143-152.
Maaswinkel, H., Jarrard, L.E. & Whishaw, I.Q. (1999) Hippocampectomized rats are impaired in homing by path
integration. Hippocampus, 9, 553-561.
MacKay, W.A. & Murphy, J.T. (1979) Cerebellar modulation of reflex gain. Prog Neurobiol, 13, 361-417.
Mackie, K. & Hille, B. (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells.
Proc Natl Acad Sci U S A, 89, 3825-3829.
Mackie, K. (2005) Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb
Exp Pharmacol, 299-325.
Mackie, K. (2008) Signaling via CNS cannabinoid receptors. Mol Cell Endocrinol, 286, S60-65.
Mackie, K., Lai, Y., Westenbroek, R. & Mitchell, R. (1995) Cannabinoids activate an inwardly rectifying
potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain
cannabinoid receptor. J Neurosci, 15, 6552-6561.
Maejima, T., Hashimoto, K., Yoshida, T., Aiba, A. & Kano, M. (2001) Presynaptic inhibition caused by
retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron, 31, 463-475.
Maguire, E.A., Gadian, D.G., Johnsrude, I.S., Good, C.D., Ashburner, J., Frackowiak, R.S. & Frith, C.D. (2000)
Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A, 97, 4398-
4403.
Maguire, E.A., Nannery, R. & Spiers, H.J. (2006) Navigation around London by a taxi driver with bilateral
hippocampal lesions. Brain, 129, 2894-2907.
Mailleux, P. & Vanderhaeghen, J.J. (1992) Distribution of neuronal cannabinoid receptor in the adult rat brain: a
comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience, 48,
655-668.
Mallet, P.E. & Beninger, R.J. (1998) The cannabinoid CB1 receptor antagonist SR141716A attenuates the
memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl),
140, 11-19.
Malone, D.T., Kearn, C.S., Chongue, L., Mackie, K. & Taylor, D.A. (2008) Effect of social isolation on CB1 and
D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience, 152, 265-272.
Mandarim-de-Lacerda, C.A., Fernandes-Santos, C. & Aguila, M.B. Image analysis and quantitative morphology.
Methods Mol Biol, 611, 211-225.
Maneuf, Y.P. & Brotchie, J.M. (1997) Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and
basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol, 120, 1397-1398.
Manton, K.G., Stallard, E. & Tolley, H. D. (1991) Limits to human life expectancy: Evidence, prospects, and
implications. Population and Development Review, 17, 603-637.
Marchalant, Y., Brothers, H.M. & Wenk, G.L. (2009) New neuron production can be increased in the
hippocampus of aged rats following cannabinoid treatment. Mol Psychiatry, 14, 1067, 1068-1069.
Marchalant, Y., Cerbai, F., Brothers, H.M. & Wenk, G.L. (2008) Cannabinoid receptor stimulation is anti-
inflammatory and improves memory in old rats. Neurobiol Aging, 29, 1894-1901.
Maren, S. & Baudry, M. (1995) Properties and mechanisms of long-term synaptic plasticity in the mammalian
brain: relationships to learning and memory. Neurobiol Learn Mem, 63, 1-18.

References 187
Maresz, K., Carrier, E.J., Ponomarev, E.D., Hillard, C.J. & Dittel, B.N. (2005) Modulation of the cannabinoid
CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem, 95, 437-445.
Markowska, A.L. (1999) Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to
alterations in the estrous cycle. J Neurosci, 19, 8122-8133.
Marsicano, G., Goodenough, S., Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S.C., Cascio, M.G.,
Gutierrez, S.O., van der Stelt, M., Lopez-Rodriguez, M.L., Casanova, E., Schutz, G., Zieglgansberger, W.,
Di Marzo, V., Behl, C. & Lutz, B. (2003) CB1 cannabinoid receptors and on-demand defense against
excitotoxicity. Science, 302, 84-88.
Marsicano, G., Wotjak, C.T., Azad, S.C., Bisogno, T., Rammes, G., Cascio, M.G., Hermann, H., Tang, J.,
Hofmann, C., Zieglgansberger, W., Di Marzo, V. & Lutz, B. (2002) The endogenous cannabinoid system
controls extinction of aversive memories. Nature, 418, 530-534.
Martin, S.J., Grimwood, P.D. & Morris, R.G. (2000) Synaptic plasticity and memory: an evaluation of the
hypothesis. Annu Rev Neurosci, 23, 649-711.
Mato, S. & Pazos, A. (2004) Influence of age, postmortem delay and freezing storage period on cannabinoid
receptor density and functionality in human brain. Neuropharmacology, 46, 716-726.
Mato, S., Chevaleyre, V., Robbe, D., Pazos, A., Castillo, P.E. & Manzoni, O.J. (2004) A single in-vivo exposure
to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci, 7, 585-586.
Mato, S., Lafourcade, M., Robbe, D., Bakiri, Y. & Manzoni, O.J. (2008) Role of the cyclic-AMP/PKA cascade
and of P/Q-type Ca++ channels in endocannabinoid-mediated long-term depression in the nucleus
accumbens. Neuropharmacology, 54, 87-94.
Matsuda, L.A., Bonner, T.I. & Lolait, S.J. (1993) Localization of cannabinoid receptor mRNA in rat brain. J
Comp Neurol, 327, 535-550.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. & Bonner, T.I. (1990) Structure of a cannabinoid
receptor and functional expression of the cloned cDNA. Nature, 346, 561-564.
Matthews, B.L., Ryu, J.H. & Bockaneck, C. (1989) Vestibular contribution to spatial orientation. Evidence of
vestibular navigation in an animal model. Acta Otolaryngol Suppl, 468, 149-154.
Maurer, C., Mergner, T., Bolha, B. & Hlavacka, F. (2000) Vestibular, visual, and somatosensory contributions to
human control of upright stance. Neurosci Lett, 281, 99-102.
Maykut, M.O. (1985) Health consequences of acute and chronic marihuana use. Prog Neuropsychopharmacol
Biol Psychiatry, 9, 209-238.
Mbongo, F., Qu'hen, C., Vidal, P.P., Tran Ba Huy, P. & de Waele, C. (2009) Role of vestibular input in triggering
and modulating postural responses in unilateral and bilateral vestibular loss patients. Audiol Neurootol, 14,
130-138.
McFarland, M.J. & Barker, E.L. (2004) Anandamide transport. Pharmacol Ther, 104, 117-135.
McFarland, M.J., Porter, A.C., Rakhshan, F.R., Rawat, D.S., Gibbs, R.A. & Barker, E.L. (2004) A role for
caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem,
279, 41991-41997.
McGauran, A.M., O'Mara, S.M. & Commins, S. (2005) Vestibular influence on water maze retention: transient
whole body rotations improve the accuracy of the cue-based retention strategy. Behav Brain Res, 158, 183-
187.

References 188
McGregor, I.S., Issakidis, C.N. & Prior, G. (1996) Aversive effects of the synthetic cannabinoid CP 55,940 in
rats. Pharmacol Biochem Behav, 53, 657-664.
McLaughlin, P.J., Winston, K., Swezey, L., Wisniecki, A., Aberman, J., Tardif, D.J., Betz, A.J., Ishiwari, K.,
Makriyannis, A. & Salamone, J.D. (2003) The cannabinoid CB1 antagonists SR 141716A and AM 251
suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol, 14, 583-
588.
McNaughton, B.L., Battaglia, F.P., Jensen, O., Moser, E.I. & Moser, M.B. (2006) Path integration and the neural
basis of the 'cognitive map'. Nat Rev Neurosci, 7, 663-678.
McPartland, J.M., Glass, M. & Pertwee, R.G. (2007) Meta-analysis of cannabinoid ligand binding affinity and
receptor distribution: interspecies differences. Br J Pharmacol, 152, 583-593.
Means, L.W., Alexander, S.R. & O'Neal, M.F. (1992) Those cheating rats: male and female rats use odor trails in
a water-escape "working memory" task. Behav Neural Biol, 58, 144-151.
Means, L.W., Hardy, W.T., Gabriel, M. & Uphold, J.D. (1971) Utilization of odor trails by rats in maze learning.
J Comp Physiol Psychol, 76, 160-164.
Mechoulam, R. & Gaoni, Y. (1967) The absolute configuration of delta-1-tetrahydrocannabinol, the major active
constituent of hashish. Tetrahedron Lett, 12, 1109-1111.
Mechoulam, R. (1970) Marijuana chemistry. Science, 168, 1159-1166.
Mechoulam, R. (1986) Cannabis as therapeutic agent. CRC Press, Boca Raton, FL.
Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N.E., Schatz, A.R., Gopher, A., Almog, S.,
Martin, B.R., Compton, D.R. & et al. (1995) Identification of an endogenous 2-monoglyceride, present in
canine gut, that binds to cannabinoid receptors. Biochem Pharmacol, 50, 83-90.
Mechoulam, R., Shani, A., Edery, H. & Grunfeld, Y. (1970) Chemical basis of hashish activity. Science, 169,
611-612.
Mergner, T., Schweigart, G., Fennell, L. & Maurer, C. (2009) Posture control in vestibular-loss patients. Ann N Y
Acad Sci, 1164, 206-215.
Meunier, M., Bachevalier, J., Mishkin, M. & Murray, E.A. (1993) Effects on visual recognition of combined and
separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci, 13, 5418-5432.
Meyers, J.R., Hu, Z., Lu, Z. & Corwin, J. T. (2009) Hair cell regeneration. In Squire, L.R. (ed) Encyclopedia of
Neuroscience. Academic Press, pp. 1005-1013.
Miettinen, R.A., Kalesnykas, G. & Koivisto, E.H. (2002) Estimation of the total number of cholinergic neurons
containing estrogen receptor-alpha in the rat basal forebrain. J Histochem Cytochem, 50, 891-902.
Miller, L.L. & Branconnier, R.J. (1983) Cannabis: effects on memory and the cholinergic limbic system. Psychol
Bull, 93, 441-456.
Mishkin, M. (1978) Memory in monkeys severely impaired by combined but not by separate removal of
amygdala and hippocampus. Nature, 273, 297-298.
Misner, D.L. & Sullivan, J.M. (1999) Mechanism of cannabinoid effects on long-term potentiation and
depression in hippocampal CA1 neurons. J Neurosci, 19, 6795-6805.
Mitrirattanakul, S., Lopez-Valdes, H.E., Liang, J., Matsuka, Y., Mackie, K., Faull, K.F. & Spigelman, I. (2007)
Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat
model of alcohol withdrawal and dependence. Alcohol Clin Exp Res, 31, 855-867.

References 189
Mochizuki, T., Okakura-Mochizuki, K., Horii, A., Yamamoto, Y. & Yamatodani, A. (1994) Histaminergic
modulation of hippocampal acetylcholine release in vivo. J Neurochem, 62, 2275-2282.
Moldrich, G. & Wenger, T. (2000) Localization of the CB1 cannabinoid receptor in the rat brain. An
immunohistochemical study. Peptides, 21, 1735-1742.
Molina-Holgado, F., Rubio-Araiz, A., Garcia-Ovejero, D., Williams, R.J., Moore, J.D., Arevalo-Martin, A.,
Gomez-Torres, O. & Molina-Holgado, E. (2007) CB2 cannabinoid receptors promote mouse neural stem
cell proliferation. Eur J Neurosci, 25, 629-634.
Morgan, N.H., Stanford, I.M. & Woodhall, G.L. (2009) Functional CB2 type cannabinoid receptors at CNS
synapses. Neuropharmacology, 57, 356-368.
Morris, R.G., Garrud, P., Rawlins, J.N. & O'Keefe, J. (1982) Place navigation impaired in rats with hippocampal
lesions. Nature, 297, 681-683.
Moser, M.B. (1999) Making more synapses: a way to store information? Cell Mol Life Sci, 55, 593-600.
Motulsky, H. (1995) The GraphPad guide to analyzing radioligand binding data www.graphpad.com. GraphPad
Software, Inc, pp. 1-18.
Mumby, D., Pinel, J. P. J. & Wood, E. R. (1990) Nonrecurring-items delayed nonmatching-to-sample in rats: A
new paradigm for testing nonspatial working memory. Psychobiology, 18, 321-326.
Mumby, D.G. (2001) Perspectives on object-recognition memory following hippocampal damage: lessons from
studies in rats. Behav Brain Res, 127, 159-181.
Munro, S., Thomas, K.L. & Abu-Shaar, M. (1993) Molecular characterization of a peripheral receptor for
cannabinoids. Nature, 365, 61-65.
Murray, E.A. & Mishkin, M. (1986) Visual recognition in monkeys following rhinal cortical ablations combined
with either amygdalectomy or hippocampectomy. J Neurosci, 6, 1991-2003.
Murray, J.B. (1986) Marijuana's effects on human cognitive functions, psychomotor functions, and personality. J
Gen Psychol, 113, 23-55.
Nakamura-Palacios, E.M., Winsauer, P.J. & Moerschbaecher, J.M. (2000) Effects of the cannabinoid ligand SR
141716A alone or in combination with delta9-tetrahydrocannabinol or scopolamine on learning in squirrel
monkeys. Behav Pharmacol, 11, 377-386.
Nakamura, E.M., da Silva, E.A., Concilio, G.V., Wilkinson, D.A. & Masur, J. (1991) Reversible effects of acute
and long-term administration of delta-9-tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol
Depend, 28, 167-175.
Nardini, M., Jones, P., Bedford, R. & Braddick, O. (2008) Development of cue integration in human navigation.
Curr Biol, 18, 689-693.
Nashner, L.M., Black, F.O. & Wall, C., 3rd (1982) Adaptation to altered support and visual conditions during
stance: patients with vestibular deficits. J Neurosci, 2, 536-544.
Nava, F., Carta, G., Battasi, A.M. & Gessa, G.L. (2000) D(2) dopamine receptors enable delta(9)-
tetrahydrocannabinol induced memory impairment and reduction of hippocampal extracellular
acetylcholine concentration. Br J Pharmacol, 130, 1201-1210.
Nava, F., Carta, G., Colombo, G. & Gessa, G.L. (2001) Effects of chronic Delta(9)-tetrahydrocannabinol
treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-
maze. Neuropharmacology, 41, 392-399.

References 190
Nawata, Y., Hiranita, T. & Yamamoto, T. A cannabinoid CB(1) receptor antagonist ameliorates impairment of
recognition memory on withdrawal from MDMA (Ecstasy). Neuropsychopharmacology, 35, 515-520.
Neitz, J. & Jacobs, G.H. (1986) Reexamination of spectral mechanisms in the rat (Rattus norvegicus). J Comp
Psychol, 100, 21-29.
Nestor, L., Roberts, G., Garavan, H. & Hester, R. (2008) Deficits in learning and memory: parahippocampal
hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage, 40, 1328-1339.
Neu, A., Foldy, C. & Soltesz, I. (2007) Postsynaptic origin of CB1-dependent tonic inhibition of GABA release
at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus.
J Physiol, 578, 233-247.
Newlands, S.D., Dara, S. & Kaufman, G.D. (2005) Relationship of static and dynamic mechanisms in
vestibuloocular reflex compensation. Laryngoscope, 115, 191-204.
Newlands, S.D., Perachio, A. A. (1990) Compensation of horizontal canal related activity in the medial
vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. Experimental Brain
Research, 82, 359-372.
Ni, X., Geller, E.B., Eppihimer, M.J., Eisenstein, T.K., Adler, M.W. & Tuma, R.F. (2004) Win 55212-2, a
cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune
encephalomyelitis model. Mult Scler, 10, 158-164.
Norre, M.E., Forrez, G. & Stevens, M. (1984) Posturography and vestibular compensation. Acta
Otorhinolaryngol Belg, 38, 619-631.
Núñez, E., Benito, C., Pazos, M.R., Barbachano, A., Fajardo, O., Gonzalez, S., Tolon, R.M. & Romero, J. (2004)
Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an
immunohistochemical study. Synapse, 53, 208-213.
Núñez, E., Benito, C., Tolon, R.M., Hillard, C.J., Griffin, W.S. & Romero, J. (2008) Glial expression of
cannabinoid CB(2) receptors and fatty acid amide hydrolase are beta amyloid-linked events in Down's
syndrome. Neuroscience, 151, 104-110.
Nyiri, G., Cserep, C., Szabadits, E., Mackie, K. & Freund, T.F. (2005) CB1 cannabinoid receptors are enriched in
the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience,
136, 811-822.
O'Keefe, J. & Dostrovsky, J. (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity
in the freely-moving rat. Brain Res, 34, 171-175.
O'Keefe, J. & Nadel, L. (1978) The hippocampus as a cognitive map. Clarendon Press, Oxford.
O'Keefe, J. (1979) A review of the hippocampal place cells. Prog Neurobiol, 13, 419-439.
O'Mara, S.M., Rolls, E.T., Berthoz, A. & Kesner, R.P. (1994) Neurons responding to whole-body motion in the
primate hippocampus. J Neurosci, 14, 6511-6523.
O'Shea, M. & Mallet, P.E. (2005) Impaired learning in adulthood following neonatal delta9-THC exposure.
Behav Pharmacol, 16, 455-461.
O'Shea, M., McGregor, I.S. & Mallet, P.E. (2006) Repeated cannabinoid exposure during perinatal, adolescent or
early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in
rats. J Psychopharmacol, 20, 611-621.
O'Shea, M., Singh, M.E., McGregor, I.S. & Mallet, P.E. (2004) Chronic cannabinoid exposure produces lasting

References 191
memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol, 18, 502-
508.
Ohno-Shosaku, T., Maejima, T. & Kano, M. (2001) Endogenous cannabinoids mediate retrograde signals from
depolarized postsynaptic neurons to presynaptic terminals. Neuron, 29, 729-738.
Ohno-Shosaku, T., Matsui, M., Fukudome, Y., Shosaku, J., Tsubokawa, H., Taketo, M.M., Manabe, T. & Kano,
M. (2003) Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde
endocannabinoid signalling in the hippocampus. Eur J Neurosci, 18, 109-116.
Ohno-Shosaku, T., Shosaku, J., Tsubokawa, H. & Kano, M. (2002) Cooperative endocannabinoid production by
neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci, 15, 953-
961.
Olabi, B., Bergquist, F. & Dutia, M.B. (2009) Rebalancing the commissural system: mechanisms of vestibular
compensation. J Vestib Res, 19, 201-207.
Olshansky, S.J., Carnes, B.A. & Cassel, C. (1990) In search of Methuselah: estimating the upper limits to human
longevity. Science, 250, 634-640.
Olton, D.S. & Papas, B.C. (1979) Spatial memory and hippocampal function. Neuropsychologia, 17, 669-682.
Olton, D.S. & Werz, M.A. (1978) Hippocampal function and behavior: spatial discrimination and response
inhibition. Physiol Behav, 20, 597-605.
Onaivi, E.S. (2006) Neuropsychobiological evidence for the functional presence and expression of cannabinoid
CB2 receptors in the brain. Neuropsychobiology, 54, 231-246.
Onaivi, E.S., Ishiguro, H., Gong, J.P., Patel, S., Perchuk, A., Meozzi, P.A., Myers, L., Mora, Z., Tagliaferro, P.,
Gardner, E., Brusco, A., Akinshola, B.E., Liu, Q.R., Hope, B., Iwasaki, S., Arinami, T., Teasenfitz, L. &
Uhl, G.R. (2006) Discovery of the presence and functional expression of cannabinoid CB2 receptors in
brain. Ann N Y Acad Sci, 1074, 514-536.
Ong, W.Y. & Mackie, K. (1999) A light and electron microscopic study of the CB1 cannabinoid receptor in
primate brain. Neuroscience, 92, 1177-1191.
Ossenkopp, K.P. & Hargreaves, E.L. (1993) Spatial learning in an enclosed eight-arm radial maze in rats with
sodium arsanilate-induced labyrinthectomies. Behav Neural Biol, 59, 253-257.
Ossenkopp, K.P., Prkacin, A. & Hargreaves, E.L. (1990) Sodium arsanilate-induced vestibular dysfunction in
rats: effects on open-field behavior and spontaneous activity in the automated digiscan monitoring system.
Pharmacol Biochem Behav, 36, 875-881.
Pacheco, M.A., Ward, S.J. & Childers, S.R. (1993) Identification of cannabinoid receptors in cultures of rat
cerebellar granule cells. Brain Res, 603, 102-110.
Palazuelos, J., Aguado, T., Egia, A., Mechoulam, R., Guzman, M. & Galve-Roperh, I. (2006) Non-psychoactive
CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J, 20, 2405-2407.
Pamplona, F.A., Bitencourt, R.M. & Takahashi, R.N. (2008) Short- and long-term effects of cannabinoids on the
extinction of contextual fear memory in rats. Neurobiol Learn Mem, 90, 290-293.
Pan, X., Ikeda, S.R. & Lewis, D.L. (1996) Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a
neuronal expression system. Mol Pharmacol, 49, 707-714.
Panikashvili, D., Simeonidou, C., Ben-Shabat, S., Hanus, L., Breuer, A., Mechoulam, R. & Shohami, E. (2001)
An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature, 413, 527-531.

References 192
Parietti-Winkler, C., Gauchard, G.C., Simon, C. & Perrin, P.P. (2010) Long-term effects of vestibular
compensation on balance control and sensory organisation after unilateral deafferentation due to vestibular
schwannoma surgery. J Neurol Neurosurg Psychiatry, 81, 934-936.
Paxinos, G., Watson, C. (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego.
Pazos, M.R., Sagredo, O. & Fernandez-Ruiz, J. (2008) The endocannabinoid system in Huntington's disease.
Curr Pharm Des, 14, 2317-2325.
Pertwee, R.G. & Ross, R.A. (2002) Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty
Acids, 66, 101-121.
Pertwee, R.G. (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther, 74, 129-180.
Pertwee, R.G. (1999) Pharmacology of cannabinoid receptor ligands. Curr Med Chem, 6, 635-664.
Pertwee, R.G. (2005a) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci, 76, 1307-
1324.
Pertwee, R.G. (2005b) Pharmacological actions of cannabinoids. Handb Exp Pharmacol, 1-51.
Pertwee, R.G. (2006) The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes
(Lond), 30 Suppl 1, S13-18.
Peruch, P., Borel, L., Gaunet, F., Thinus-Blanc, G., Magnan, J. & Lacour, M. (1999) Spatial performance of
unilateral vestibular defective patients in nonvisual versus visual navigation. J Vestib Res, 9, 37-47.
Peterson, B.W. & Richmond, F.J. (1988) Control of Head Movement. Oxford University Press., New York.
Petitet, F., Jeantaud, B., Capet, M. & Doble, A. (1997) Interaction of brain cannabinoid receptors with guanine
nucleotide binding protein: a radioligand binding study. Biochem Pharmacol, 54, 1267-1270.
Pettit, D.A., Harrison, M.P., Olson, J.M., Spencer, R.F. & Cabral, G.A. (1998) Immunohistochemical localization
of the neural cannabinoid receptor in rat brain. J Neurosci Res, 51, 391-402.
Piomelli, D. (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci, 4, 873-884.
Piomelli, D., Beltramo, M., Glasnapp, S., Lin, S.Y., Goutopoulos, A., Xie, X.Q. & Makriyannis, A. (1999)
Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad
Sci U S A, 96, 5802-5807.
Pistis, M., Muntoni, A.L., Pillolla, G. & Gessa, G.L. (2002) Cannabinoids inhibit excitatory inputs to neurons in
the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur J Neurosci, 15, 1795-1802.
Pitler, T.A. & Alger, B.E. (1992) Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal
pyramidal cells. J Neurosci, 12, 4122-4132.
Polissidis, A., Chouliara, O., Galanopoulos, A., Marselos, M., Papadopoulou-Daifoti, Z. & Antoniou, K. (2009)
Behavioural and dopaminergic alterations induced by a low dose of WIN 55,212-2 in a conditioned place
preference procedure. Life Sci, 85, 248-254.
Pope, H.G., Jr. & Yurgelun-Todd, D. (1996) The residual cognitive effects of heavy marijuana use in college
students. JAMA, 275, 521-527.
Porter, A.C., Sauer, J.M., Knierman, M.D., Becker, G.W., Berna, M.J., Bao, J., Nomikos, G.G., Carter, P.,
Bymaster, F.P., Leese, A.B. & Felder, C.C. (2002) Characterization of a novel endocannabinoid,
virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther, 301, 1020-1024.
Porter, J.D., Pellis, S.M. & Meyer, M.E. (1990) An open-field activity analysis of labyrinthectomized rats.
Physiol Behav, 48, 27-30.

References 193
Pozzo, T., Berthoz, A., Lefort, L. & Vitte, E. (1991) Head stabilization during various locomotor tasks in humans.
II. Patients with bilateral peripheral vestibular deficits. Exp Brain Res, 85, 208-217.
Prather, P.L., Martin, N.A., Breivogel, C.S. & Childers, S.R. (2000) Activation of cannabinoid receptors in rat
brain by WIN 55212-2 produces coupling to multiple G protein alpha-subunits with different potencies.
Mol Pharmacol, 57, 1000-1010.
Price, D.A., Martinez, A.A., Seillier, A., Koek, W., Acosta, Y., Fernandez, E., Strong, R., Lutz, B., Marsicano, G.,
Roberts, J.L. & Giuffrida, A. (2009) WIN55,212-2, a cannabinoid receptor agonist, protects against
nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's
disease. Eur J Neurosci, 29, 2177-2186.
Pruessner, J.C., Kirschbaum, C., Meinlschmid, G. & Hellhammer, D.H. (2003) Two formulas for computation of
the area under the curve represent measures of total hormone concentration versus time-dependent change.
Psychoneuroendocrinology, 28, 916-931.
Quinn, H.R., Matsumoto, I., Callaghan, P.D., Long, L.E., Arnold, J.C., Gunasekaran, N., Thompson, M.R.,
Dawson, B., Mallet, P.E., Kashem, M.A., Matsuda-Matsumoto, H., Iwazaki, T. & McGregor, I.S. (2008)
Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual
cognitive deficits and changes in hippocampal protein expression following exposure.
Neuropsychopharmacology, 33, 1113-1126.
Quirk, G.J., Muller, R.U. & Kubie, J.L. (1990) The firing of hippocampal place cells in the dark depends on the
rat's recent experience. J Neurosci, 10, 2008-2017.
Qureshi, J., Saady, M., Cardounel, A. & Kalimi, M. (1998) Identification and characterization of a novel
synthetic cannabinoid CP 55,940 binder in rat brain cytosol. Mol Cell Biochem, 181, 21-27.
Ramirez, B.G., Blazquez, C., Gomez del Pulgar, T., Guzman, M. & de Ceballos, M.L. (2005) Prevention of
Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial
activation. J Neurosci, 25, 1904-1913.
Ranganathan, M. & D'Souza, D.C. (2006) The acute effects of cannabinoids on memory in humans: a review.
Psychopharmacology (Berl), 188, 425-444.
Redfern, M.S., Talkowski, M.E., Jennings, J.R. & Furman, J.M. (2004) Cognitive influences in postural control
of patients with unilateral vestibular loss. Gait Posture, 19, 105-114.
Reibaud, M., Obinu, M.C., Ledent, C., Parmentier, M., Bohme, G.A. & Imperato, A. (1999) Enhancement of
memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol, 379, R1-2.
Rhodes, K.J. & Trimmer, J.S. (2006) Antibodies as valuable neuroscience research tools versus reagents of mass
distraction. J Neurosci, 26, 8017-8020.
Rice, J. (2007) Mathematical statistics and data analysis. Duxbury Press, Belmont, California.
Richfield, E.K. & Herkenham, M. (1994) Selective vulnerability in Huntington's disease: preferential loss of
cannabinoid receptors in lateral globus pallidus. Ann Neurol, 36, 577-584.
Rinaldi-Carmona, M., Barth, F., Heaulme, M., Shire, D., Calandra, B., Congy, C., Martinez, S., Maruani, J.,
Neliat, G., Caput, D. & et al. (1994) SR141716A, a potent and selective antagonist of the brain
cannabinoid receptor. FEBS Lett, 350, 240-244.
Ris, L. & Godaux, E. (1998) Neuronal activity in the vestibular nuclei after contralateral or bilateral
labyrinthectomy in the alert guinea pig. J Neurophysiol, 80, 2352-2367.

References 194
Risey, J. & Briner, W. (1990) Dyscalculia in patients with vertigo. J Vestib Res, 1, 31-37.
Rivers, J.R. & Ashton, J.C. (2010) The development of cannabinoid CBII receptor agonists for the treatment of
central neuropathies. Cent Nerv Syst Agents Med Chem, 10, 47-64.
Robbe, D. & Buzsaki, G. (2009) Alteration of theta timescale dynamics of hippocampal place cells by a
cannabinoid is associated with memory impairment. J Neurosci, 29, 12597-12605.
Robbe, D., Alonso, G. & Manzoni, O.J. (2003) Exogenous and endogenous cannabinoids control synaptic
transmission in mice nucleus accumbens. Ann N Y Acad Sci, 1003, 212-225.
Robbe, D., Kopf, M., Remaury, A., Bockaert, J. & Manzoni, O.J. (2002) Endogenous cannabinoids mediate
long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A, 99, 8384-8388.
Robbe, D., Montgomery, S.M., Thome, A., Rueda-Orozco, P.E., McNaughton, B.L. & Buzsaki, G. (2006)
Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci, 9,
1526-1533.
Robinson, L., Goonawardena, A.V., Pertwee, R.G., Hampson, R.E. & Riedel, G. (2007) The synthetic
cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br J
Pharmacol, 151, 688-700.
Robinson, L., Hinder, L., Pertwee, R.G. & Riedel, G. (2003) Effects of delta9-THC and WIN-55,212-2 on place
preference in the water maze in rats. Psychopharmacology (Berl), 166, 40-50.
Rodgers, R.J., Evans, P.M. & Murphy, A. (2005) Anxiogenic profile of AM-251, a selective cannabinoid CB1
receptor antagonist, in plus-maze-naive and plus-maze-experienced mice. Behav Pharmacol, 16, 405-413.
Rodriguez de Fonseca, F., Del Arco, I., Bermudez-Silva, F.J., Bilbao, A., Cippitelli, A. & Navarro, M. (2005)
The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol, 40, 2-14.
Rodriguez de Fonseca, F., Del Arco, I., Martin-Calderon, J.L., Gorriti, M.A. & Navarro, M. (1998) Role of the
endogenous cannabinoid system in the regulation of motor activity. Neurobiol Dis, 5, 483-501.
Rodriguez de Fonseca, F., Gorriti, M.A., Fernandez-Ruiz, J.J., Palomo, T. & Ramos, J.A. (1994) Downregulation
of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol
Biochem Behav, 47, 33-40.
Rolls, E.T. (1996) A theory of hippocampal function in memory. Hippocampus, 6, 601-620.
Roloff, A.M. & Thayer, S.A. (2009) Modulation of excitatory synaptic transmission by Delta 9-
tetrahydrocannabinol switches from agonist to antagonist depending on firing rate. Mol Pharmacol, 75,
892-900.
Romero, J., Berrendero, F., Manzanares, J., Perez, A., Corchero, J., Fuentes, J.A., Fernandez-Ruiz, J.J. & Ramos,
J.A. (1998) Time-course of the cannabinoid receptor down-regulation in the adult rat brain caused by
repeated exposure to delta9-tetrahydrocannabinol. Synapse, 30, 298-308.
Romero, J., Garcia, L., Fernandez-Ruiz, J.J., Cebeira, M. & Ramos, J.A. (1995) Changes in rat brain cannabinoid
binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-
tetrahydrocannabinol. Pharmacol Biochem Behav, 51, 731-737.
Romero, J., Lastres-Becker, I., de Miguel, R., Berrendero, F., Ramos, J.A. & Fernandez-Ruiz, J. (2002) The
endogenous cannabinoid system and the basal ganglia. biochemical, pharmacological, and therapeutic
aspects. Pharmacol Ther, 95, 137-152.
Ronesi, J., Gerdeman, G.L. & Lovinger, D.M. (2004) Disruption of endocannabinoid release and striatal long-

References 195
term depression by postsynaptic blockade of endocannabinoid membrane transport. J Neurosci, 24, 1673-
1679.
Roozendaal, B., Phillips, R.G., Power, A.E., Brooke, S.M., Sapolsky, R.M. & McGaugh, J.L. (2001) Memory
retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat
Neurosci, 4, 1169-1171.
Rossier, J., Haeberli, C. & Schenk, F. (2000) Auditory cues support place navigation in rats when associated with
a visual cue. Behav Brain Res, 117, 209-214.
Rothblat, L.A. & Hayes, L.L. (1987) Short-term object recognition memory in the rat: nonmatching with trial-
unique junk stimuli. Behav Neurosci, 101, 587-590.
Routtenberg, A. (1984) The CA3 pyramidal cell in the hippocampus: Site of intrinsic expression and extrinsic
control of memory formation. In Squire, L.R. & Butters, N. (ed) Neuropsychology of Memory. Guilford
Press, New York, pp. 536-546.
Rubino, T., Realini, N., Braida, D., Alberio, T., Capurro, V., Vigano, D., Guidali, C., Sala, M., Fasano, M. &
Parolaro, D. (2009a) The depressive phenotype induced in adult female rats by adolescent exposure to
THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox
Res, 15, 291-302.
Rubino, T., Realini, N., Braida, D., Guidi, S., Capurro, V., Vigano, D., Guidali, C., Pinter, M., Sala, M.,
Bartesaghi, R. & Parolaro, D. (2009b) Changes in hippocampal morphology and neuroplasticity induced
by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus, 19,
763-772.
Rubino, T., Vigano, D., Massi, P. & Parolaro, D. (2000a) Changes in the cannabinoid receptor binding, G protein
coupling, and cyclic AMP cascade in the CNS of rats tolerant to and dependent on the synthetic
cannabinoid compound CP55,940. J Neurochem, 75, 2080-2086.
Rubino, T., Vigano, D., Massi, P., Spinello, M., Zagato, E., Giagnoni, G. & Parolaro, D. (2000b) Chronic delta-9-
tetrahydrocannabinol treatment increases cAMP levels and cAMP-dependent protein kinase activity in
some rat brain regions. Neuropharmacology, 39, 1331-1336.
Rueda-Orozco, P.E., Soria-Gomez, E., Montes-Rodriguez, C.J., Martinez-Vargas, M., Galicia, O., Navarro, L. &
Prospero-Garcia, O. (2008) A potential function of endocannabinoids in the selection of a navigation
strategy by rats. Psychopharmacology (Berl), 198, 565-576.
Russell, N.A., Horii, A., Smith, P.F., Darlington, C.L. & Bilkey, D.K. (2003a) Bilateral peripheral vestibular
lesions produce long-term changes in spatial learning in the rat. J Vestib Res, 13, 9-16.
Russell, N.A., Horii, A., Smith, P.F., Darlington, C.L. & Bilkey, D.K. (2003b) Long-term effects of permanent
vestibular lesions on hippocampal spatial firing. J Neurosci, 23, 6490-6498.
Russell, N.A., Horii, A., Smith, P.F., Darlington, C.L. & Bilkey, D.K. (2006) Lesions of the vestibular system
disrupt hippocampal theta rhythm in the rat. J Neurophysiol, 96, 4-14.
Ryberg, E., Vu, H.K., Larsson, N., Groblewski, T., Hjorth, S., Elebring, T., Sjogren, S. & Greasley, P.J. (2005)
Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett, 579,
259-264.
Ryu, J.H., McCabe, B. F. (1976) Central vestibular compensation. Effect of bilateral labyrinthectomy on neural
activity in the medial vestibular nucleus. Archives of Otolaryngology, 102, 71-76.

References 196
Sakurai, Y. (1990) Hippocampal cells have behavioral correlates during the performance of an auditory working
memory task in the rat. Behav Neurosci, 104, 253-263.
Sakurai, Y. (1994) Involvement of auditory cortical and hippocampal neurons in auditory working memory and
reference memory in the rat. J Neurosci, 14, 2606-2623.
Sakurai, Y. (2002) Coding of auditory temporal and pitch information by hippocampal individual cells and cell
assemblies in the rat. Neuroscience, 115, 1153-1163.
Saman, Y., Bamiou, D.E. & Gleeson, M. (2009) A contemporary review of balance dysfunction following
vestibular schwannoma surgery. Laryngoscope, 119, 2085-2093.
Sang, F.Y., Jauregui-Renaud, K., Green, D.A., Bronstein, A.M. & Gresty, M.A. (2006)
Depersonalisation/derealisation symptoms in vestibular disease. J Neurol Neurosurg Psychiatry, 77, 760-
766.
Sanudo-Pena, M.C. & Walker, J.M. (1998) A novel neurotransmitter system involved in the control of motor
behavior by the basal ganglia. Ann N Y Acad Sci, 860, 475-479.
Sanudo-Pena, M.C., Patrick, S.L., Patrick, R.L. & Walker, J.M. (1996) Effects of intranigral cannabinoids on
rotational behavior in rats: interactions with the dopaminergic system. Neurosci Lett, 206, 21-24.
Saper, C.B. & Sawchenko, P.E. (2003) Magic peptides, magic antibodies: guidelines for appropriate controls for
immunohistochemistry. J Comp Neurol, 465, 161-163.
Saunders, R.C., Murray, E.A. & Mishkin, M. (1984) Further evidence that amygdala and hippocampus
contribute equally to recognition memory. Neuropsychologia, 22, 785-796.
Save, E., Cressant, A., Thinus-Blanc, C. & Poucet, B. (1998) Spatial firing of hippocampal place cells in blind
rats. J Neurosci, 18, 1818-1826.
Sawant, P.M., Holland, P.T., Mountfort, D.O. & Kerr, D.S. (2008) In vivo seizure induction and pharmacological
preconditioning by domoic acid and isodomoic acids A, B and C. Neuropharmacology, 55, 1412-1418.
Saxon, D.W., Anderson, J.H. & Beitz, A.J. (2001) Transtympanic tetrodotoxin alters the VOR and Fos labeling in
the vestibular complex. Neuroreport, 12, 3051-3055.
Schaeppi, U., Krinke, G., FitzGerald, R.E. & Classen, W. (1991) Impaired tunnel-maze behavior in rats with
sensory lesions: vestibular and auditory systems. Neurotoxicology, 12, 445-454.
Schatz, A.R., Lee, M., Condie, R.B., Pulaski, J.T. & Kaminski, N.E. (1997) Cannabinoid receptors CB1 and CB2:
a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl
Pharmacol, 142, 278-287.
Schautzer, F., Hamilton, D., Kalla, R., Strupp, M. & Brandt, T. (2003) Spatial memory deficits in patients with
chronic bilateral vestibular failure. Ann N Y Acad Sci, 1004, 316-324.
Schirmer, M., Kaiser, A., Lessenich, A., Lindemann, S., Fedrowitz, M., Gernert, M. & Loscher, W. (2007c)
Auditory and vestibular defects and behavioral alterations after neonatal administration of streptomycin to
Lewis rats: Similarities and differences to the circling (ci2/ci2) Lewis rat mutant. Brain Res, 1155, 179-195.
Schirmer, M., Lessenich, A., Lindemann, S. & Loscher, W. (2007a) Marked differences in response to dopamine
receptor antagonism in two rat mutants, ci2 and ci3, with lateralized rotational behavior. Behav Brain Res,
180, 218-225.
Schirmer, M., Nobrega, J.N., Harrison, S.J. & Loscher, W. (2007b) Alterations in dopamine D3 receptors in the
circling (ci3) rat mutant. Neuroscience, 144, 1462-1469.

References 197
Schlicker, E. & Kathmann, M. (2001) Modulation of transmitter release via presynaptic cannabinoid receptors.
Trends Pharmacol Sci, 22, 565-572.
Schlicker, E., Timm, J., Zentner, J. & Gothert, M. (1997) Cannabinoid CB1 receptor-mediated inhibition of
noradrenaline release in the human and guinea-pig hippocampus. Naunyn Schmiedebergs Arch Pharmacol,
356, 583-589.
Schmidt-Koenig, K. (1965) Current problems in bird orientation. Adv Study Behav, 1, 217-278.
Schneider, M. & Koch, M. (2002) The cannabinoid agonist WIN 55,212-2 reduces sensorimotor gating and
recognition memory in rats. Behav Pharmacol, 13, 29-37.
Schneider, M. & Koch, M. (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs
sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats.
Neuropsychopharmacology, 28, 1760-1769.
Schneider, M. & Koch, M. (2005) Deficient social and play behavior in juvenile and adult rats after neonatal
cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology, 30, 944-957.
Schneider, M. & Koch, M. (2007) The effect of chronic peripubertal cannabinoid treatment on deficient object
recognition memory in rats after neonatal mPFC lesion. Eur Neuropsychopharmacol, 17, 180-186.
Schneider, M., Schomig, E. & Leweke, F.M. (2008) Acute and chronic cannabinoid treatment differentially
affects recognition memory and social behavior in pubertal and adult rats. Addict Biol, 13, 345-357.
Scoville, W.B. & Milner, B. (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol
Neurosurg Psychiatry, 20, 11-21.
Selley, D.E., Stark, S., Sim, L.J. & Childers, S.R. (1996) Cannabinoid receptor stimulation of guanosine-5'-O-(3-
[35S]thio)triphosphate binding in rat brain membranes. Life Sci, 59, 659-668.
Serpa, A., Ribeiro, J.A. & Sebastiao, A.M. (2009) Cannabinoid CB(1) and adenosine A(1) receptors
independently inhibit hippocampal synaptic transmission. Eur J Pharmacol, 623, 41-46.
Sharp, P.E., Blair, H.T., Etkin, D. & Tzanetos, D.B. (1995) Influences of vestibular and visual motion
information on the spatial firing patterns of hippocampal place cells. J Neurosci, 15, 173-189.
Shen, M. & Thayer, S.A. (1998) The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-
mediated and direct pathways in cultured rat hippocampal neurons. Brain Res, 783, 77-84.
Shen, M., Piser, T.M., Seybold, V.S. & Thayer, S.A. (1996) Cannabinoid receptor agonists inhibit glutamatergic
synaptic transmission in rat hippocampal cultures. J Neurosci, 16, 4322-4334.
Shire, D., Carillon, C., Kaghad, M., Calandra, B., Rinaldi-Carmona, M., Le Fur, G., Caput, D. & Ferrara, P.
(1995) An amino-terminal variant of the central cannabinoid receptor resulting from alternative splicing. J
Biol Chem, 270, 3726-3731.
Shoaib, M. (2008) The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-
seeking behaviour in rats. Neuropharmacology, 54, 438-444.
Sim-Selley, L.J., Brunk, L.K. & Selley, D.E. (2001) Inhibitory effects of SR141716A on G-protein activation in
rat brain. Eur J Pharmacol, 414, 135-143.
Sim, L.J., Hampson, R.E., Deadwyler, S.A. & Childers, S.R. (1996) Effects of chronic treatment with delta9-
tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J
Neurosci, 16, 8057-8066.
Sim, L.J., Selley, D.E. & Childers, S.R. (1995) In vitro autoradiography of receptor-activated G proteins in rat

References 198
brain by agonist-stimulated guanylyl 5'-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci U S A,
92, 7242-7246.
Simantov, R., Crispino, M., Hoe, W., Broutman, G., Tocco, G., Rothstein, J.D. & Baudry, M. (1999) Changes in
expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced
seizure activity. Brain Res Mol Brain Res, 65, 112-123.
Sink, K.S., McLaughlin, P.J., Wood, J.A., Brown, C., Fan, P., Vemuri, V.K., Peng, Y., Olszewska, T., Thakur,
G.A., Makriyannis, A., Parker, L.A. & Salamone, J.D. (2008) The novel cannabinoid CB1 receptor neutral
antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of
nausea in rats. Neuropsychopharmacology, 33, 946-955.
Sirkin, D.W., Precht, W. & Courjon, J.H. (1984) Initial, rapid phase of recovery from unilateral vestibular lesion
in rat not dependent on survival of central portion of vestibular nerve. Brain Res, 302, 245-256.
Sjostrom, P.J., Turrigiano, G.G. & Nelson, S.B. (2003) Neocortical LTD via coincident activation of presynaptic
NMDA and cannabinoid receptors. Neuron, 39, 641-654.
Skaggs, W.E. (1993) An information-theoretic approach to deciphering the hippocampal code. In Hanson, S.J.,
Cowan, J. D. & Giles, C. L. (ed) Advances in neural information processing systems. Morgan Kaufmann,
San Mateo, CA, pp. P 1030-1037.
Skaper, S.D., Buriani, A., Dal Toso, R., Petrelli, L., Romanello, S., Facci, L. & Leon, A. (1996) The ALIAmide
palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate
paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci U S A, 93, 3984-3989.
Smith, P.F. & Curthoys, I.S. (1988a) Neuronal activity in the contralateral medial vestibular nucleus of the
guinea pig following unilateral labyrinthectomy. Brain Res, 444, 295-307.
Smith, P.F. & Curthoys, I.S. (1988b) Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea
pig following unilateral labyrinthectomy. Brain Res, 444, 308-319.
Smith, P.F. & Curthoys, I.S. (1989) Mechanisms of recovery following unilateral labyrinthectomy: a review.
Brain Res Brain Res Rev, 14, 155-180.
Smith, P.F. (1997) Vestibular-hippocampal interactions. Hippocampus, 7, 465-471.
Smith, P.F., Brandt, T., Strupp, M., Darlington, C.L. & Zheng, Y. (2009) Balance before reason in rats and
humans. Ann N Y Acad Sci, 1164, 127-133.
Smith, P.F., Darlington, C.L. & Curthoys, I.S. (1986) The effect of visual deprivation on vestibular compensation
in the guinea pig. Brain Res, 364, 195-198.
Smith, P.F., Darlington, C.L. & Zheng, Y. (2010) Move it or lose it--is stimulation of the vestibular system
necessary for normal spatial memory? Hippocampus, 20, 36-43.
Smith, P.F., Geddes, L.H., Baek, J.-H., Darlington, C.L., and Zheng, Y. (2010, in press) Modulation of memory
in humans by vestibular lesions and galvanic vestibular stimulation. Front Neur.
Smith, P.F., Horii, A., Russell, N., Bilkey, D.K., Zheng, Y., Liu, P., Kerr, D.S. & Darlington, C.L. (2005a) The
effects of vestibular lesions on hippocampal function in rats. Prog Neurobiol, 75, 391-405.
Smith, P.F., Zheng, Y., Goddard, M. & Darlington, C. L. (2007) Cognitive Disorders Research Trends. Nova
Science Publishers.
Smith, P.F., Zheng, Y., Horii, A. & Darlington, C.L. (2005b) Does vestibular damage cause cognitive dysfunction
in humans? J Vestib Res, 15, 1-9.

References 199
Solinas, M. & Goldberg, S.R. (2005) Motivational effects of cannabinoids and opioids on food reinforcement
depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology, 30,
2035-2045.
Solowij, N. (1995) Do cognitive impairments recover following cessation of cannabis use? Life Sci, 56, 2119-
2126.
Solowij, N., Stephens, R.S., Roffman, R.A., Babor, T., Kadden, R., Miller, M., Christiansen, K., McRee, B. &
Vendetti, J. (2002) Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA, 287,
1123-1131.
Song, C. & Howlett, A.C. (1995) Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci, 56,
1983-1989.
Spiers, H.J., Burgess, N., Maguire, E.A., Baxendale, S.A., Hartley, T., Thompson, P.J. & O'Keefe, J. (2001)
Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a
virtual town. Brain, 124, 2476-2489.
Squire, L.R. (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans.
Psychol Rev, 99, 195-231.
Squire, L.R. & Zola-Morgan, S. (1991) The medial temporal lobe memory system. Science, 253, 1380-1386.
Squire, L.R. & Zola, S.M. (1996) Structure and function of declarative and nondeclarative memory systems.
Proc Natl Acad Sci U S A, 93, 13515-13522.
Squire, L.R., Stark, C.E. & Clark, R.E. (2004) The medial temporal lobe. Annu Rev Neurosci, 27, 279-306.
Staab, J.P. & Ruckenstein, M.J. (2003) Which comes first? Psychogenic dizziness versus otogenic anxiety.
Laryngoscope, 113, 1714-1718.
Stackman, R.W. & Herbert, A.M. (2002) Rats with lesions of the vestibular system require a visual landmark for
spatial navigation. Behav Brain Res, 128, 27-40.
Stackman, R.W. & Taube, J.S. (1997) Firing properties of head direction cells in the rat anterior thalamic nucleus:
dependence on vestibular input. J Neurosci, 17, 4349-4358.
Stackman, R.W., Clark, A.S. & Taube, J.S. (2002) Hippocampal spatial representations require vestibular input.
Hippocampus, 12, 291-303.
Steffenach, H.A., Sloviter, R.S., Moser, E.I. & Moser, M.B. (2002) Impaired retention of spatial memory after
transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proc Natl Acad Sci U S
A, 99, 3194-3198.
Stella, N., Schweitzer, P. & Piomelli, D. (1997) A second endogenous cannabinoid that modulates long-term
potentiation. Nature, 388, 773-778.
Stubley-Weatherly, L., Harding, J.W. & Wright, J.W. (1996) Effects of discrete kainic acid-induced hippocampal
lesions on spatial and contextual learning and memory in rats. Brain Res, 716, 29-38.
Stupien, G., Florian, C. & Roullet, P. (2003) Involvement of the hippocampal CA3-region in acquisition and in
memory consolidation of spatial but not in object information in mice. Neurobiol Learn Mem, 80, 32-41.
Suàrez, J., Bermudez-Silva, F.J., Mackie, K., Ledent, C., Zimmer, A., Cravatt, B.F. & de Fonseca, F.R. (2008)
Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and
functionally related nuclei. J Comp Neurol, 509, 400-421.
Suàrez, J., Llorente, R., Romero-Zerbo, S.Y., Mateos, B., Bermudez-Silva, F.J., de Fonseca, F.R. & Viveros, M.P.

References 200
(2009) Early maternal deprivation induces gender-dependent changes on the expression of hippocampal
CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus, 19, 623-632.
Suenaga, T. & Ichitani, Y. (2008) Effects of hippocampal administration of a cannabinoid receptor agonist WIN
55,212-2 on spontaneous object and place recognition in rats. Behav Brain Res, 190, 248-252.
Suenaga, T., Kaku, M. & Ichitani, Y. (2008) Effects of intrahippocampal cannabinoid receptor agonist and
antagonist on radial maze and T-maze delayed alternation performance in rats. Pharmacol Biochem Behav,
91, 91-96.
Sugiura, T. & Waku, K. (2002) Cannabinoid receptors and their endogenous ligands. J Biochem, 132, 7-12.
Sugiura, T. (2009) Physiological roles of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand.
Biofactors, 35, 88-97.
Sugiura, T., Kishimoto, S., Oka, S. & Gokoh, M. (2006) Biochemistry, pharmacology and physiology of 2-
arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res, 45, 405-446.
Sugiura, T., Kobayashi, Y., Oka, S. & Waku, K. (2002) Biosynthesis and degradation of anandamide and 2-
arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty
Acids, 66, 173-192.
Sugiura, T., Kondo, S., Kishimoto, S., Miyashita, T., Nakane, S., Kodaka, T., Suhara, Y., Takayama, H. & Waku,
K. (2000) Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the
physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various
cannabinoid receptor ligands in HL-60 cells. J Biol Chem, 275, 605-612.
Sugiura, T., Kondo, S., Sukagawa, A., Nakane, S., Shinoda, A., Itoh, K., Yamashita, A. & Waku, K. (1995) 2-
Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res
Commun, 215, 89-97.
Sutherland, B.A., Rahman, R.M., Clarkson, A.N., Shaw, O.M., Nair, S.M. & Appleton, I. (2009) Cerebral heme
oxygenase 1 and 2 spatial distribution is modulated following injury from hypoxia-ischemia and middle
cerebral artery occlusion in rats. Neurosci Res, 65, 326-334.
Sutherland, R.J. & Dyck, R. H. (1984) Place navigation by rats in a swimming pool. Can J Pschol, 38, 322-347.
Suzuki, M., Kitano, H., Ito, R., Kitanishi, T., Yazawa, Y., Ogawa, T., Shiino, A. & Kitajima, K. (2001) Cortical
and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance
imaging. Brain Res Cogn Brain Res, 12, 441-449.
Szabo, B., Siemes, S. & Wallmichrath, I. (2002) Inhibition of GABAergic neurotransmission in the ventral
tegmental area by cannabinoids. Eur J Neurosci, 15, 2057-2061.
Szabo, B., Wallmichrath, I., Mathonia, P. & Pfreundtner, C. (2000) Cannabinoids inhibit excitatory
neurotransmission in the substantia nigra pars reticulata. Neuroscience, 97, 89-97.
Tagliaferro, P., Javier Ramos, A., Onaivi, E.S., Evrard, S.G., Lujilde, J. & Brusco, A. (2006) Neuronal
cytoskeleton and synaptic densities are altered after a chronic treatment with the cannabinoid receptor
agonist WIN 55,212-2. Brain Res, 1085, 163-176.
Takahashi, K.A. & Castillo, P.E. (2006) The CB1 cannabinoid receptor mediates glutamatergic synaptic
suppression in the hippocampus. Neuroscience, 139, 795-802.
Takahashi, R.N., Pamplona, F.A. & Fernandes, M.S. (2005) The cannabinoid antagonist SR141716A facilitates
memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett, 380, 270-275.

References 201
Talpos, J.C., Dias, R., Bussey, T.J. & Saksida, L.M. (2008) Hippocampal lesions in rats impair learning and
memory for locations on a touch-sensitive computer screen: the "ASAT" task. Behav Brain Res, 192, 216-
225.
Taylor, H.G. (1998) Analysis of the medical use of marijuana and its societal implications. J Am Pharm Assoc
(Wash), 38, 220-227.
Tchernichovski, O. & Benjamini, Y. (1998) The dynamics of long-term exploration in the rat. Part II. An
analytical model of the kinematic structure of rat exploratory behavior. Biol Cybern, 78, 433-440.
Terranova, J.P., Michaud, J.C., Le Fur, G. & Soubrie, P. (1995) Inhibition of long-term potentiation in rat
hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of
CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol, 352, 576-579.
Terranova, J.P., Storme, J.J., Lafon, N., Perio, A., Rinaldi-Carmona, M., Le Fur, G. & Soubrie, P. (1996)
Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716.
Psychopharmacology (Berl), 126, 165-172.
Terrazas, A., Krause, M., Lipa, P., Gothard, K.M., Barnes, C.A. & McNaughton, B.L. (2005) Self-motion and the
hippocampal spatial metric. J Neurosci, 25, 8085-8096.
Thomas, B.F., Gilliam, A.F., Burch, D.F., Roche, M.J. & Seltzman, H.H. (1998) Comparative receptor binding
analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther, 285, 285-292.
Tsou, K., Brown, S., Sanudo-Pena, M.C., Mackie, K. & Walker, J.M. (1998) Immunohistochemical distribution
of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience, 83, 393-411.
Tsou, K., Mackie, K., Sanudo-Pena, M.C. & Walker, J.M. (1999) Cannabinoid CB1 receptors are localized
primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation.
Neuroscience, 93, 969-975.
Turrigiano, G.G. (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell, 135, 422-435.
Turu, G. & Hunyady, L. (2010) Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol, 44, 75-
85.
Valverde, O., Karsak, M. & Zimmer, A. (2005) Analysis of the endocannabinoid system by using CB1
cannabinoid receptor knockout mice. Handb Exp Pharmacol, 117-145.
van der Meer, M.A., Knierim, J.J., Yoganarasimha, D., Wood, E.R. & van Rossum, M.C. (2007) Anticipation in
the rodent head direction system can be explained by an interaction of head movements and vestibular
firing properties. J Neurophysiol, 98, 1883-1897.
van der Stelt, M., Mazzola, C., Esposito, G., Matias, I., Petrosino, S., De Filippis, D., Micale, V., Steardo, L.,
Drago, F., Iuvone, T. & Di Marzo, V. (2006) Endocannabinoids and beta-amyloid-induced neurotoxicity in
vivo: effect of pharmacological elevation of endocannabinoid levels. Cell Mol Life Sci, 63, 1410-1424.
Van Sickle, M.D., Duncan, M., Kingsley, P.J., Mouihate, A., Urbani, P., Mackie, K., Stella, N., Makriyannis, A.,
Piomelli, D., Davison, J.S., Marnett, L.J., Di Marzo, V., Pittman, Q.J., Patel, K.D. & Sharkey, K.A. (2005)
Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310, 329-
332.
Vander Wall, S.B. (1982) An experimental analysis of cache recovery in Clark's nutcracker. Anim Behav, 30, 84-
94.
Varma, N., Carlson, G.C., Ledent, C. & Alger, B.E. (2001) Metabotropic glutamate receptors drive the

References 202
endocannabinoid system in hippocampus. J Neurosci, 21, RC188.
Varvel, S.A. & Lichtman, A.H. (2002) Evaluation of CB1 receptor knockout mice in the Morris water maze. J
Pharmacol Exp Ther, 301, 915-924.
Varvel, S.A., Anum, E.A. & Lichtman, A.H. (2005) Disruption of CB(1) receptor signaling impairs extinction of
spatial memory in mice. Psychopharmacology (Berl), 179, 863-872.
Vinod, K.Y., Arango, V., Xie, S., Kassir, S.A., Mann, J.J., Cooper, T.B. & Hungund, B.L. (2005) Elevated levels
of endocannabinoids and CB1 receptor-mediated G-protein signaling in the prefrontal cortex of alcoholic
suicide victims. Biol Psychiatry, 57, 480-486.
Vitte, E., Derosier, C., Caritu, Y., Berthoz, A., Hasboun, D. & Soulie, D. (1996) Activation of the hippocampal
formation by vestibular stimulation: a functional magnetic resonance imaging study. Exp Brain Res, 112,
523-526.
Waespe, W., Schwarz, U., Wolfenburger, M. (1992) Firing characteristics of vestibular nuclei neurons in the alert
monkey after bilateral vestibular neurectomy. Experimental Brain Research, 89, 311-322.
Wallace, D.G. & Whishaw, I.Q. (2003) NMDA lesions of Ammon's horn and the dentate gyrus disrupt the direct
and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the
hippocampus in dead reckoning. Eur J Neurosci, 18, 513-523.
Wallace, M.J., Blair, R.E., Falenski, K.W., Martin, B.R. & DeLorenzo, R.J. (2003) The endogenous cannabinoid
system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp
Ther, 307, 129-137.
Wallace, D.G., Gorny, B. & Whishaw, I.Q. (2002a) Rats can track odors, other rats, and themselves: implications
for the study of spatial behavior. Behav Brain Res, 131, 185-192.
Wallace, D.G., Hines, D.J. & Whishaw, I.Q. (2002b) Quantification of a single exploratory trip reveals
hippocampal formation mediated dead reckoning. J Neurosci Methods, 113, 131-145.
Wallace, D.G., Hines, D.J., Pellis, S.M. & Whishaw, I.Q. (2002c) Vestibular information is required for dead
reckoning in the rat. J Neurosci, 22, 10009-10017.
Wallmichrath, I. & Szabo, B. (2002) Analysis of the effect of cannabinoids on GABAergic neurotransmission in
the substantia nigra pars reticulata. Naunyn Schmiedebergs Arch Pharmacol, 365, 326-334.
Walsh, S., Mnich, K., Mackie, K., Gorman, A.M., Finn, D.P. & Dowd, E. (2010) Loss of cannabinoid CB1
receptor expression in the 6-hydroxydopamine-induced nigrostriatal terminal lesion model of Parkinson's
disease in the rat. Brain Res Bull, 81, 543-548.
Wang, S.J. (2003) Cannabinoid CB1 receptor-mediated inhibition of glutamate release from rat hippocampal
synaptosomes. Eur J Pharmacol, 469, 47-55.
Watanabe, S. & Yoshida, M. (2007) Auditory cued spatial learning in mice. Physiol Behav, 92, 906-910.
Wegener, N., Kuhnert, S., Thuns, A., Roese, R. & Koch, M. (2008) Effects of acute systemic and intra-cerebral
stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats.
Psychopharmacology (Berl), 198, 375-385.
Weinstein, A., Brickner, O., Lerman, H., Greemland, M., Bloch, M., Lester, H., Chisin, R., Sarne, Y., Mechoulam,
R., Bar-Hamburger, R., Freedman, N. & Even-Sapir, E. (2008) A study investigating the acute dose-
response effects of 13 mg and 17 mg Delta 9- tetrahydrocannabinol on cognitive-motor skills, subjective
and autonomic measures in regular users of marijuana. J Psychopharmacol, 22, 441-451.

References 203
West, M.J. & Gundersen, H.J. (1990) Unbiased stereological estimation of the number of neurons in the human
hippocampus. J Comp Neurol, 296, 1-22.
West, M.J., Slomianka, L. & Gundersen, H.J. (1991) Unbiased stereological estimation of the total number of
neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec, 231, 482-497.
Whishaw, I.Q. & Brooks, B.L. (1999) Calibrating space: exploration is important for allothetic and idiothetic
navigation. Hippocampus, 9, 659-667.
Whishaw, I.Q. & Gorny, B. (1999) Path integration absent in scent-tracking fimbria-fornix rats: evidence for
hippocampal involvement in "sense of direction" and "sense of distance" using self-movement cues. J
Neurosci, 19, 4662-4673.
Whishaw, I.Q. & Maaswinkel, H. (1998) Rats with fimbria-fornix lesions are impaired in path integration: a role
for the hippocampus in "sense of direction". J Neurosci, 18, 3050-3058.
Whishaw, I.Q. & Tomie, J.A. (1997) Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat
food carrying task. Behav Brain Res, 89, 87-97.
Whishaw, I.Q. (1985) Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies.
Physiol Behav, 35, 139-143.
Whishaw, I.Q. (1998) Spatial mapping takes time. Hippocampus, 8, 122-130.
Whishaw, I.Q., Coles, B.L. & Bellerive, C.H. (1995) Food carrying: a new method for naturalistic studies of
spontaneous and forced alternation. J Neurosci Methods, 61, 139-143.
Whishaw, I.Q., Hines, D.J. & Wallace, D.G. (2001) Dead reckoning (path integration) requires the hippocampal
formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark
(idiothetic) tests. Behav Brain Res, 127, 49-69.
Whishaw, I.Q., Oddie, S.D., McNamara, R.K., Harris, T.L. & Perry, B.S. (1990) Psychophysical methods for
study of sensory-motor behavior using a food-carrying (hoarding) task in rodents. J Neurosci Methods, 32,
123-133.
Wiener, S.I., Korshunov, V.A., Garcia, R. & Berthoz, A. (1995) Inertial, substratal and landmark cue control of
hippocampal CA1 place cell activity. Eur J Neurosci, 7, 2206-2219.
Wilkinson, D., Nicholls, S., Pattenden, C., Kilduff, P. & Milberg, W. (2008) Galvanic vestibular stimulation
speeds visual memory recall. Exp Brain Res, 189, 243–248.
Wilkinson, D., Zubko, O., DeGutis, J., Milberg, W. & Potter, J. (2010) Improvement of a figure copying deficit
during subsensory galvanic vestibular stimulation. J. Neuropsychol, 4, 107-118.
Williams, S.M., Bryan-Lluka, L.J. & Pow, D.V. (2005) Quantitative analysis of immunolabeling for serotonin
and for glutamate transporters after administration of imipramine and citalopram. Brain Res, 1042, 224-
232.
Wilson, R.I. & Nicoll, R.A. (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal
synapses. Nature, 410, 588-592.
Wilson, V.J. & Melvill Jones, G. (1979) Mammalian vestibular physiology. Plenum Press, New York and London.
Winer, B.J., Brown, D. R. & Michels, K. M. (ed) (1991) Statistical Principles in Experimental Design. McGraw
Hill, New York.
Winsauer, P.J., Lambert, P. & Moerschbaecher, J.M. (1999) Cannabinoid ligands and their effects on learning and
performance in rhesus monkeys. Behav Pharmacol, 10, 497-511.

References 204
Winters, B.D., Forwood, S.E., Cowell, R.A., Saksida, L.M. & Bussey, T.J. (2004) Double dissociation between
the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial
memory: heterogeneity of function within the temporal lobe. J Neurosci, 24, 5901-5908.
Winters, B.D., Saksida, L.M. & Bussey, T.J. (2008) Object recognition memory: neurobiological mechanisms of
encoding, consolidation and retrieval. Neurosci Biobehav Rev, 32, 1055-1070.
Wise, L.E., Thorpe, A.J. & Lichtman, A.H. (2009) Hippocampal CB(1) receptors mediate the memory impairing
effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology, 34, 2072-2080.
Wolf, S.A., Bick-Sander, A., Fabel, K., Leal-Galicia, P., Tauber, S., Ramirez-Rodriguez, G., Muller, A., Melnik,
A., Waltinger, T.P., Ullrich, O. & Kempermann, G. (2010) Cannabinoid receptor CB1 mediates baseline
and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal, 8,
12.
Wolff, M.C. & Leander, J.D. (2003) SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a
delayed radial maze task. Eur J Pharmacol, 477, 213-217.
Wong, D.F., Kuwabara, H., Horti, A.G., Raymont, V., Brasic, J., Guevara, M., Ye, W., Dannals, R.F., Ravert, H.T.,
Nandi, A., Rahmim, A., Ming, J.E., Grachev, I., Roy, C. & Cascella, N. (2010) Quantification of cerebral
cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand
[(11)C]OMAR. Neuroimage.
Yakushin, S.B., Raphan, T., Buttner-Ennever, J.A., Suzuki, J. & Cohen, B. (2005) Spatial properties of central
vestibular neurons of monkeys after bilateral lateral canal nerve section. J Neurophysiol, 94, 3860-3871.
Yamanaka, T., Him, A., Cameron, S.A. & Dutia, M.B. (2000) Rapid compensatory changes in GABA receptor
efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol, 523 Pt 2, 413-424.
Yim, T.T., Hong, N.S., Ejaredar, M., McKenna, J.E. & McDonald, R.J. (2008) Post-training CB1 cannabinoid
receptor agonist activation disrupts long-term consolidation of spatial memories in the hippocampus.
Neuroscience, 151, 929-936.
Yoshida, T., Hashimoto, K., Zimmer, A., Maejima, T., Araishi, K. & Kano, M. (2002) The cannabinoid CB1
receptor mediates retrograde signals for depolarization-induced suppression of inhibition in cerebellar
Purkinje cells. J Neurosci, 22, 1690-1697.
Zajonc, T.P. & Roland, P.S. (2005) Vertigo and motion sickness. Part I: vestibular anatomy and physiology. Ear
Nose Throat J, 84, 581-584.
Zar, J.H. (4th ed) (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, New Jersey.
Zennou-Azogui, Y., Borel, L., Lacour, M., Ez-Zaher, L. & Ouaknine, M. (1993) Recovery of head postural
control following unilateral vestibular neurectomy in the cat. Neck muscle activity and neuronal correlates
in Deiters' nuclei. Acta Otolaryngol Suppl, 509, 1-19.
Zhang, M., Martin, B.R., Adler, M.W., Razdan, R.K., Ganea, D. & Tuma, R.F. (2008) Modulation of the balance
between cannabinoid CB(1) and CB(2) receptor activation during cerebral ischemic/reperfusion injury.
Neuroscience, 152, 753-760.
Zhang, M., Martin, B.R., Adler, M.W., Razdan, R.K., Jallo, J.I. & Tuma, R.F. (2007) Cannabinoid CB(2)
receptor activation decreases cerebral infarction in a mouse focal ischemia/reperfusion model. J Cereb
Blood Flow Metab, 27, 1387-1396.
Zhang, R., Ashton, J., Horii, A., Darlington, C.L. & Smith, P.F. (2005a) Immunocytochemical and stereological

References 205
analysis of GABA(B) receptor subunit expression in the rat vestibular nucleus following unilateral
vestibular deafferentation. Brain Res, 1037, 107-113.
Zhang, R., Smith, P.F. & Darlington, C.L. (2005b) Immunocytochemical and stereological study of
glucocorticoid receptors in rat medial vestibular nucleus neurons and the effects of unilateral vestibular
deafferentation. Acta Otolaryngol, 125, 1258-1264.
Zhao, W., Cavallaro, S., Gusev, P. & Alkon, D.L. (2000) Nonreceptor tyrosine protein kinase pp60c-src in spatial
learning: synapse-specific changes in its gene expression, tyrosine phosphorylation, and protein-protein
interactions. Proc Natl Acad Sci U S A, 97, 8098-8103.
Zheng, Y., Balabhadrapatruni, S., Masumura, C., Munro, O., Darlington, C.L. & Smith, P.F. (2009a) Bilateral
vestibular deafferentation causes deficits in a 5-choice serial reaction time task in rats. Behav Brain Res,
203, 113-117.
Zheng, Y., Darlington, C.L. & Smith, P.F. (2004) Bilateral labyrinthectomy causes long-term deficit in object
recognition in rat. Neuroreport, 15, 1913-1916.
Zheng, Y., Darlington, C.L. & Smith, P.F. (2006) Impairment and recovery on a food foraging task following
unilateral vestibular deafferentation in rats. Hippocampus, 16, 368-378.
Zheng, Y., Goddard, M., Darlington, C.L. & Smith, P.F. (2007) Bilateral vestibular deafferentation impairs
performance in a spatial forced alternation task in rats. Hippocampus, 17, 253-256.
Zheng, Y., Goddard, M., Darlington, C.L. & Smith, P.F. (2008) Effects of bilateral vestibular deafferentation on
anxiety-related behaviours in Wistar rats. Behav Brain Res, 193, 55-62.
Zheng, Y., Goddard, M., Darlington, C.L. & Smith, P.F. (2009b) Long-term deficits on a foraging task after
bilateral vestibular deafferentation in rats. Hippocampus, 19, 480-486.
Zheng, Y., Horii, A., Appleton, I., Darlington, C.L. & Smith, P.F. (2001) Damage to the vestibular inner ear
causes long-term changes in neuronal nitric oxide synthase expression in the rat hippocampus.
Neuroscience, 105, 1-5.
Zheng, Y., Kerr, D.S., Darlington, C.L. & Smith, P.F. (2003) Unilateral inner ear damage results in lasting
changes in hippocampal CA1 field potentials in vitro. Hippocampus, 13, 873-878.
Zheng, Y., Mason-Parker, S.E., Logan, B., Darlington, C.L., Smith, P.F. & Abraham, W.C. (2010) Hippocampal
synaptic transmission and LTP in vivo are intact following bilateral vestibular deafferentation in the rat.
Hippocampus, 20, 461-468.
Zimmer, A., Zimmer, A.M., Hohmann, A.G., Herkenham, M. & Bonner, T.I. (1999) Increased mortality,
hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A, 96,
5780-5785.
Zimmerberg, B., Glick, S.D. & Jarvik, M.E. (1971) Impairment of recent memory by marihuana and THC in
rhesus monkeys. Nature, 233, 343-345.
Zingler, V.C., Weintz, E., Jahn, K., Mike, A., Huppert, D., Rettinger, N., Brandt, T. & Strupp, M. (2008) Follow-
up of vestibular function in bilateral vestibulopathy. J Neurol Neurosurg Psychiatry, 79, 284-288.
Zola, S.M., Squire, L.R., Teng, E., Stefanacci, L., Buffalo, E.A. & Clark, R.E. (2000) Impaired recognition
memory in monkeys after damage limited to the hippocampal region. J Neurosci, 20, 451-463.
Zola-Morgan, S. & Squire, L.R. (1985) Medial temporal lesions in monkeys impair memory on a variety of tasks
sensitive to human amnesia. Behav Neurosci, 99, 22-34.

References 206
Zola-Morgan, S. & Squire, L.R. (1986) Memory impairment in monkeys following lesions limited to the
hippocampus. Behav Neurosci, 100, 155-160.
Zola-Morgan, S., Squire, L.R., Clower, R.P. & Rempel, N.L. (1993) Damage to the perirhinal cortex exacerbates
memory impairment following lesions to the hippocampal formation. J Neurosci, 13, 251-265.
zu Eulenburg, P., Stoeter, P. & Dieterich, M. (2010) Voxel-based morphometry depicts central compensation
after vestibular neuritis. Ann Neurol, 68, 241-249.

The New Beginning 206
This unforgettable journey has taught me
Perseverance to endure difficult times,
humility in gaining knowledge,
Diligence to work consistently.
But by the grace of God I am what I am, and His grace to me was not without effect.
<1 Corinthians 15:10>
Related Documents